Effects of housing conditions on health and gut microbiome of female cynomolgus monkeys and improvement of welfare by checking menstruation under socially housed condition
- PMID: 39897812
- PMCID: PMC11786677
- DOI: 10.1016/j.heliyon.2025.e41912
Effects of housing conditions on health and gut microbiome of female cynomolgus monkeys and improvement of welfare by checking menstruation under socially housed condition
Abstract
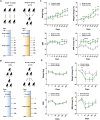
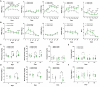
Laboratory non-human primates (NHPs) are commonly subjected to social deprivation in various scientific researches. However, the impact of social deprivation on gut microbiome remains largely unknown. We examined the health status and gut microbiota of female cynomolgus monkeys housed in isolation or social conditions and found that social deprivation brought adverse effects to monkeys by inhibiting their growth, remodeling the immune status, and decreasing the level of beneficial biochemical parameters. 16S rRNA gene sequencing revealed that the gut microbial composition and function differed between grouped and isolated monkeys. Specifically, grouping the single-caged young monkeys to socially housed condition could decrease the relative abundance of Firmicutes and increase the relative abundance of Bacteroidetes, while separating the socially housed middle-aged monkeys into single cages showed the opposite trend. Besides, training female monkeys to detect menstruation under socially-housed condition could increase their body weight change and adjusting their immune status, thus attenuating the adverse effects of separating them to single cages. Our results verified the significant role of grouping in mitigating adverse health and microbiota alterations caused by isolation in female cynomolgus monkeys and emphasized the importance of training NHPs to cooperate with experimental procedures under socially housed condition, which could not only improve the welfare of cynomolgus monkeys but also enhance the accuracy and reliability of scientific results.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Stress in cynomolgus monkeys (Macaca fascicularis) subjected to long-distance transport and simulated transport housing conditions.Stress. 2008 Nov;11(6):467-76. doi: 10.1080/10253890801903359. Stress. 2008. PMID: 18609299
-
Telemetric assessment of social and single housing: Evaluation of electrocardiographic intervals in jacketed cynomolgus monkeys.J Pharmacol Toxicol Methods. 2015 Sep-Oct;75:38-43. doi: 10.1016/j.vascn.2015.05.001. Epub 2015 May 8. J Pharmacol Toxicol Methods. 2015. PMID: 25959881
-
Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys.Atherosclerosis. 1989 May;77(1):69-76. doi: 10.1016/0021-9150(89)90011-7. Atherosclerosis. 1989. PMID: 2719764
-
Metagenomic and network analysis revealed wide distribution of antibiotic resistance genes in monkey gut microbiota.Microbiol Res. 2022 Jan;254:126895. doi: 10.1016/j.micres.2021.126895. Epub 2021 Oct 23. Microbiol Res. 2022. PMID: 34742104 Review.
-
Social behavior and gender in biomedical investigations using monkeys: studies in atherogenesis.Lab Anim Sci. 1991 Aug;41(4):334-43. Lab Anim Sci. 1991. PMID: 1658480 Review.
References
-
- Dunphy-Doherty F., O'Mahony S.M., Peterson V.L., O'Sullivan O., Crispie F., Cotter P.D., Wigmore P., King M.V., Cryan J.F., Fone K.C.F. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 2018;68:261–273. doi: 10.1016/j.bbi.2017.10.024. - DOI - PubMed
LinkOut - more resources
Full Text Sources

