Spinal neuromodulation using ultra low frequency waveform inhibits sensory signaling to the thalamus and preferentially reduces aberrant firing of thalamic neurons in a model of neuropathic pain
- PMID: 39897953
- PMCID: PMC11783389
- DOI: 10.3389/fnins.2024.1512950
Spinal neuromodulation using ultra low frequency waveform inhibits sensory signaling to the thalamus and preferentially reduces aberrant firing of thalamic neurons in a model of neuropathic pain
Abstract
Introduction: Many forms of chronic pain remain refractory to existing pharmacotherapies and electrical neuromodulation. We have recently reported the clinical efficacy of a novel form of analgesic electrical neuromodulation that uses ultra low frequency (ULF™) biphasic current and studied its effects on sensory nerve fibers. Here, we show that in anesthetized rats, epidural ULF current reversibly inhibits activation of neurons in the thalamus receiving sensory spinothalamic input.
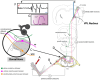
Methods: In naïve, neuropathic and sham-operated rats, recordings of ongoing and evoked activity were made from thalamic neurons, targeting the ventral posterolateral (VPL) nucleus.
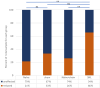
Results: Responses to electrical stimulation of hind limb receptive fields were reduced in 25 of 32 (78%) neurons tested with lumbar epidural ULF neuromodulation. Cells preferentially responsive to low intensity stimulation were more likely to be found than cells responding to a range of stimulus intensities, or high intensity only; and low threshold responses were more likely to be inhibited by ULF than high threshold responses. On-going activity unrelated to hindlimb stimulation, observed in 17 of 39 neurons in naïve animals (44%), was reduced by lumbar epidural ULF current in only 3 of 14 (21%) neurons tested with ULF. By contrast, in rats with a well-characterized neuropathic injury, spinal nerve ligation (SNL), we found a much higher incidence of on-going activity in thalamic neurons: 53 of 55 neurons (96%) displayed firing unrelated to hindlimb stimulation. In this group, ULF current reduced thalamic neurone discharge rate in 19 of 29 (66%) neurons tested. In sham-operated animals, the incidence of such activity in thalamic neurons and the effect of ULF current were not significantly different from the naïve group.
Discussion: We conclude firstly that ULF current can acutely and reversibly interrupt signaling between sensory afferent fibers and relay neurons of the thalamus. Second, ongoing activity of thalamic neurons increases dramatically in the early stages following neuropathic injury. Third, this novel form of neuromodulation preferentially attenuates pathological thalamic activity in this neuropathic model compared to normal activity in naïve and sham-operated animals. This study, therefore, demonstrates that epidural ULF current can reduce nerve injury-related abnormal activity reaching the brain. These findings help advance understanding of possible mechanisms for the analgesic effects of ULF neuromodulation.
Keywords: ULF™ neuromodulation; inhibition; neuropathic; pain; spinal cord stimulation; thalamus.
Copyright © 2025 Jones, Matthews, Lempka, Verma, Harris and McMahon.
Conflict of interest statement
MJ, LM, and SL participate in research funded by Presidio Medical Inc. SL holds stock in Presidio Medical Inc. and is a member of the scientific advisory board. NV and JH are employees of Presidio Medical Inc. Presidio Medical Inc. is a medical device company commercializing ULF neuromodulation. MJ is CEO of Zenith Neurotech Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Presidio Medical Inc. The funder had the following involvement in the study: Conceptualization, Formal analysis, Methodology, Writing – review & editing.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

