Optimizing the management of psoriasis in patients with skin of color: A Canadian Delphi consensus
- PMID: 39898016
- PMCID: PMC11783118
- DOI: 10.1016/j.jdin.2024.09.015
Optimizing the management of psoriasis in patients with skin of color: A Canadian Delphi consensus
Abstract
Background: There is limited evidence on treating psoriasis patients with skin of color (SOC), contributing to disparities in accessing appropriate care for these patients.
Objectives: This study aimed to develop consensus statements defining SOC terminology and addressing needs to optimize the clinical management of psoriasis in patients with SOC.
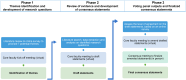
Methods: Using the modified Delphi methodology 16 Canadian dermatologists with expertise in psoriasis developed consensus statements. Four core faculty members drove the content of the study, and 12 additional panel members were consulted to vote and provide consensus on the content produced by the core faculty. At a final meeting, the full panel revised and voted on the final consensus statements.
Results: The exercise resulted in 11 consensus statements on SOC terminology, as well as 5 primary and 4 secondary statements on clinical presentation and differential diagnosis, and treatment guidelines based on evidence and expert opinion. Four additional consensus statements on current assessment tools and access to care were developed based solely on expert opinion.
Limitations: The available evidence was limited, low quality, and inappropriate for formal quality assessment.
Conclusions: The consensus statements developed in this study may provide valuable guidance to the dermatology community treating psoriasis patients with SOC.
Keywords: Canadian Delphi consensus; disparities in psoriasis management; diversity; equity; psoriasis; skin of color.
© 2024 by the American Academy of Dermatology, Inc. Published by Elsevier Inc.
Conflict of interest statement
Dr Yadav received honoraria from AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bioderma, BI, BMS, Byrdie, Galderma, Incyte, Janssen, Johnson & Johnson, Leo, Eli Lilly, L’Oreal, Medexus, Novartis, Pfizer, Sanofi-Regeneron, Sun Pharma, and UCB for advisory board and speaker services and for her participation in this study. Dr Rao received honoraria from Janssen for advisory board and speaker services and for his participation in this study. Dr Yeung received honoraria from AbbVie, Amgen, Anacor, Astellas, Arcutis, Bausche, Baxalta, Boehringer Ingelheim, BMS, Celgene, Centocor, Coherus, Dermira, Eli Lilly, Forward, Galderma, Incyte, Janssen, Leo, Medimmune, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Takeda, UCB, and Xenon for advisory board and speaker services and for his participation in this study. Dr Miller-Monthrope received honoraria from AbbVie, Bausch Health, Galderma, Incyte, Janssen, Novartis, Sanofi-Regeron, Sun Pharma, and UCB for advisory board and speaker services and for her participation in this study. Dr Metelitsa received honoraria from Abbvie, Amgen, Bausch, Boehringer Ingelheim, BMS, Celgene, Eli Lilly, Galderma, Incyte, Janssen, Leo, Norvartis, Pfizer, Sanofi Genzyme, Sun Pharma, and UCB for advisory board and speaker services and for his participation in this study. Dr Ogunyemi received honoraria from AbbVie, Janssen, Novartis, Sun Pharma, Pfizer and UCB for advisory board and speaker services and for his participation in this study. Dr Lynde received honoraria from AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Cipher, Dermavant, Eli Lilly, Fresnius Kabi, Galderma, GSK, Incyte, Innovaderm, Intega Skin, Janssen, Kyowa Kirin, La Roche Posay, LEO Pharma, L'Oreal, Medexus, MedX, Merck, Novartis, P&G, Pediapharm, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sandoz, Sentrex, SunPharma, TEVA, Tribute, UCB, Valeant, Viatris, Volo Health for advisory board and speaker services and for his participation in this study. Dr Han received honoraria from Abbvie, Amgen, Arcutis, Bausch Health, Celgene, Galderma, Janssen, Leo Pharma, Eli Lilly, Novartis, Sanofi Genzyme, Sun Pharma, UCB, and XYON for advisory board and speaker services and for her participation in this study. Dr Adam received honoraria from AbbVie, Arcutis, Amgen, Actelion, Arcutis, Bausch Health, Boehringer Ingelheim, BMS, Celgene, Coherus, Dermira, Dermavant, Eli Lilly, Galderma, Incyte, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Reistone, Regeneron, Sanofi Genzyme, Sun Pharma and UCB for advisory board and speaker services and for his participation in this study. Dr Purdy received honoraria from Abbvie, Amgen, Arcutis, Boerhinger-ingleheim, BMS, Bausch Health, Eli Lilly, Incyte, Janssen, Leo, Novartis, Pfizer, Recordati, Sanofi, Sun Pharma, and UCB for advisory board and speaker services and for her participation in this study. Dr Mitsos received honoraria from AbbVie, Amgen, Aralez, Arcutis, Bausch Health, Bioderma, BMS, Galderma, Janssen, Johnson & Johnson, Leo, Eli Lilly, L’Oreal, Medexus, Novartis, Pfizer, Sanofi-Regeneron, Sun Pharma, and UCB for advisory board and speaker services and for her participation in this study. Dr Joseph received honoraria from Abbvie, Amgen, Arcutis, Bausch, Boehringer Ingelheim, BMS, Celgene, Eli Lilly, Galderma, Incyte, Janssen, Leo, L’Oreal, Norvartis, Pfizer, Sanofi Genzyme, Sun Pharma, and UCB for advisory board and speaker services and for her participation in this study. Dr Sauder received honoraria from Amgen, AbbVie, Bausch Health, Boehringer Ingelheim, Bristol-Myers-Squibb, Janssen, LEO Pharmaceuticals, Novartis, Sun Pharmaceuticals, UCB Canada, and Viatris for advisory board and speaker services and for his participation in this study. Dr Grewal received honoraria from AbbVie, Amgen, Anacor, Arcutis, Arena Pharmaceuticals, Avillion, Bausch Health/Valeant, Boehringer Ingelheim, BMS, Celgene, Cipher, Dermavant, Dermira, Eli Lilly, Galderma, GSK, Incyte, Innovaderm, J&J/Janssen, Leo Pharma, Med Plan, Meiji Seika Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi-Aventis/Genzyme, Sandoz, Sun Pharmaceuticals, Takeda, UCB, and Vitae for advisory board and speaker services and for his participation in this study. Dr Asiniwasis received honoraria from Abbvie, Arcutis, Bausch, Boehringer-Ingleheim, Chronicle Companies, Galderma, Incyte, Janssen, Leo, Eli Lilly, L'Oreal, Medexus, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and WoundPedia for advisory board and speaker services and for her participation in this study. Dr Langley received honoraria from AbbVie, Amgen, Bausch Health, BI, BMS, Janssen, Leo, Eli Lilly, Novartis, Pfizer, Sanofi-Regeneron, Sun Pharma, and UCB for advisory board and speaker services and for his participation in this study.
Figures
References
-
- Walter K. Psoriasis. JAMA. 2022;327(19):1936. - PubMed
-
- Levy A.R., Davie A.M., Brazier N.C., et al. Economic burden of moderate to severe plaque psoriasis in Canada. Int J Dermatol. 2012;51(12):1432–1440. - PubMed
-
- Ferguson J.E., Seger E.W., White J., McMichael A. Racial/ethnic differences in treatment efficacy and safety for moderate-to-severe plaque psoriasis: a systematic review. Arch Dermatol Res. 2023;315(1):41–50. - PubMed
LinkOut - more resources
Full Text Sources