MERS-CoV spike vaccine-induced N-terminal domain-specific antibodies are more protective than receptor binding domain-specific antibodies
- PMID: 39898019
- PMCID: PMC11783452
- DOI: 10.1016/j.isci.2024.111632
MERS-CoV spike vaccine-induced N-terminal domain-specific antibodies are more protective than receptor binding domain-specific antibodies
Abstract
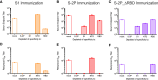
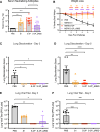
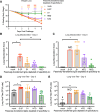
The COVID-19 pandemic underscores the need to prepare for future emerging coronavriuses (CoVs) by understanding the principles behind effective CoV vaccine design such as protective immunity and antibody responses. To study which epitopes and subdomains contribute to in vivo protection, we utilized the prefusion-stabilized spike protein of MERS-CoV, MERS S-2P, as a vaccine immunogen. Vaccination with MERS S-2P elicited both receptor-binding domain (RBD)- and non-RBD-specific antibodies, including N-terminal domain (NTD)-specific G2-and CDC2-A2-like antibodies. Intriguingly, the immunogen MERS S-2P_ΔRBD, MERS S-2P with the RBDs removed, protects comparably to S1 and S-2P immunogens against MERS-CoV challenge. Moreover, passive transfer studies of polyclonal IgG from MERS S-2P immunized mice depleted of subdomain-specific antibodies demonstrated that non-RBD antibodies protected more than non-NTD antibodies. Altogether, these findings illustrate that in-vivo protection is not solely driven by RBD-specific antibodies and highlights the importance of targeting non-RBD sites in future CoV vaccine designs.
Keywords: Immunity; Immunology; Virology.
© 2024 The Author(s).
Conflict of interest statement
N.W., K.S.C-H, M.K., A.W., B.S.G., and J.S.M. are inventors on a US patent entitled “Prefusion Coronavirus Spike Proteins and Their Use.” L.W., W.S., W.-P.K., M.K., J.R.M., and B.S.G. are inventors on a US patent application entitled “Middle East Respiratory Syndrome Coronavirus Immunogens, Antibodies, and Their Use.”
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

