Snapshots of Pseudomonas aeruginosa SOS response reveal structural requisites for LexA autoproteolysis
- PMID: 39898034
- PMCID: PMC11787620
- DOI: 10.1016/j.isci.2024.111726
Snapshots of Pseudomonas aeruginosa SOS response reveal structural requisites for LexA autoproteolysis
Abstract
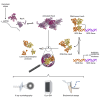
Antimicrobial resistance poses a severe threat to human health and Pseudomonas aeruginosa stands out among the pathogens responsible for this emergency. The SOS response to DNA damage is crucial in bacterial evolution, influencing resistance development and adaptability in challenging environments, especially under antibiotic exposure. Recombinase A (RecA) and the transcriptional repressor LexA are the key players that orchestrate this process, determining either the silencing or the active transcription of the genes under their control. By integrating state-of-the-art structural approaches with in vitro binding and functional assays, we elucidated the molecular events activating the SOS response in P. aeruginosa, focusing on the RecA-LexA interaction. Our findings identify the conserved determinants and strength of the interactions that allow RecA to trigger LexA autocleavage and inactivation. These results provide the groundwork for designing novel antimicrobial strategies and exploring the potential translation of Escherichia coli-derived approaches, to address the implications of P. aeruginosa infections.
Keywords: Biological sciences; Biophysics; Microbiology; Natural sciences.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

