Extracellular vesicles contribute to the beneficial effects of exercise training in APP/PS1 mice
- PMID: 39898049
- PMCID: PMC11787611
- DOI: 10.1016/j.isci.2025.111752
Extracellular vesicles contribute to the beneficial effects of exercise training in APP/PS1 mice
Abstract
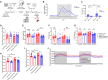
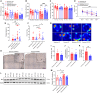
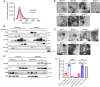
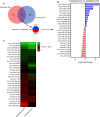
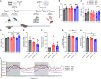
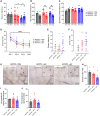
Exercise improves cognitive function in Alzheimer's disease (AD) via mechanism that are not fully clear. Here, we first examined the effect of voluntary exercise training (VET) on energy metabolism and cognitive function in the APP/PS1 transgenic mouse (Tg) model of familial AD. Next, we profiled extracellular vesicles (EVs) and examined whether they may play a role in the protective effects of VET via intranasal administration of EVs, purified from the blood of sedentary (sEV) and/or acutely exercised (eEV) donor wild-type mice into APP/PS1Tg mice. We show that VET reduced resting energy expenditure (REE) and improved cognition in APP/PS1 Tg mice. Administration of eEV, but not sEV, also reduced REE, but had no effect on cognition. Taken together, these data show that exercise is effective intervention to improve symptoms of AD in APP/PS1Tg mice. In addition, eEVs mediate some of these effects, implicating EVs in the treatment of age-related neurodegenerative diseases.
Keywords: Cell biology; Molecular physiology; Neuroscience.
© 2025 The Authors.
Conflict of interest statement
M.A.F. is a shareholder and scientific advisor for N-Gene Pharmaceuticals. M.A.F. is the founder and shareholder of Celesta Therapeutics.
Figures

References
-
- Nations U . United Nations; 2016. World Population Ageing 2015 Highlights.
-
- Alzheimer's A Alzheimer's disease facts and figures. Alzheimer's Dement. 2015;11:332. - PubMed
-
- Doorduijn A.S., Visser M., van de Rest O., Kester M.I., de Leeuw F.A., Boesveldt S., Fieldhouse J.L.P., van den Heuvel E.G.H.M., Teunissen C.E., Scheltens P., et al. Associations of AD biomarkers and cognitive performance with nutritional status: the NUDAD project. Nutrients. 2019;11:1161. - PMC - PubMed
-
- Puranen T.M., Pietila S.E., Pitkala K.H., Kautiainen H., Raivio M., Eloniemi-Sulkava U., Jyvakorpi S.K., Suominen M. Caregivers’ male gender is associated with poor nutrient intake in AD families (NuAD-trial) J. Nutr. Health Aging. 2014;18:672–676. - PubMed
-
- Tabet N., Mantle D., Walker Z., Orrell M. Higher fat and carbohydrate intake in dementia patients is associated with increased blood glutathione peroxidase activity. Int. Psychogeriatr. 2005;17:91–98. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

