Citrus Flavonoids as Antimicrobials
- PMID: 39898883
- PMCID: PMC12168191
- DOI: 10.1002/cbdv.202403210
Citrus Flavonoids as Antimicrobials
Abstract
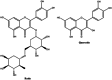
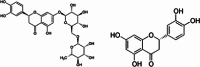
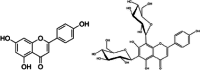
Citrus flavonoids are highly bioactive compounds exerting numerous health benefits including anticancer, antioxidant, antimicrobial, anti-inflammatory, mitoprotective, and neuroprotective activity. Research on their broad-scope bioactivity experienced a renaissance in the early 2000s, and further accelerated after COVID-19, including research on their antimicrobial properties. Summarizing selected research achievements on the antimicrobial activity of the main Citrus flavonoids, this study aims to provide a unified picture on the antimicrobial properties of these valued compounds that will hopefully assist in the development of flavonoid-based antimicrobials, including antibacterial treatments suitable for clinical use minimizing antimicrobial resistance.
Keywords: antimicrobial resistance; citrus flavonoids; diosmin; hesperidin; naringenin; polymethoxyflavones.
© 2025 The Author(s). Chemistry & Biodiversity published by Wiley‐VHCA AG.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
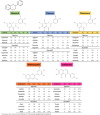
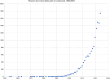

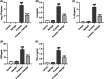
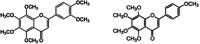




References
-
- Search within, “All Fields” Carried Out on scopus.Com, With the Query “Citrus Flavonoids”, up to December 31, 2024. January 31, 2025.
-
- Tripoli E., La Guardia M., Giammanco S., Majo D. D., and Giammanco M., “Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review,” Food Chemistry 104 (2007): 466–479, 10.1016/j.foodchem.2006.11.054. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

