Prevalence of HSV Genomic Signatures Among Acanthamoeba Hosts and Contaminated Lens Cases: A Molecular and Clinical Study
- PMID: 39898908
- PMCID: PMC11798336
- DOI: 10.1167/iovs.66.2.4
Prevalence of HSV Genomic Signatures Among Acanthamoeba Hosts and Contaminated Lens Cases: A Molecular and Clinical Study
Abstract
Purpose: To document the presence of herpes simplex virus (HSV) genomic signatures among Acanthamoeba hosts recovered from patients with Acanthamoeba keratitis (AK) and in contaminated lens cases.
Methods: We used a combination of PCR sequencing and shotgun metagenomics to detect and confirm the presence of HSV genomic signatures in Acanthamoeba hosts and lens cases. Clinical outcomes were correlated with the prevalence of host HSV signatures.
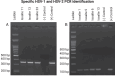
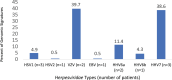
Results: HSV genomic signatures were detected in 26% (n = 6) of Acanthamoeba hosts recovered from patients with culture confirmed AK. HSV-1 and HSV-2 or both were identified in 33%, 50%, and 17% of isolates, respectively. Fifty-two percent of patients (12/23) were misdiagnosed initially as presenting with HSV keratitis. Patients with HSV-positive Acanthamoeba isolates had a mean best-corrected visual acuity of 1.43 LogMAR at diagnosis and 0.53 LogMAR at follow-up, compared with 1.85 and 0.92 LogMAR, respectively, in HSV-negative cases. Contact lens use was identified as a risk factor in 83% of 18 patients. We detected 46,597 viral signatures in 5 of 14 contaminated lens cases (35.7%). Distribution included 33% bacteriophages, 8.2% giant viruses, 4.1% nonhuman Herpesviridae, and 0.39% human Herpesviridae. Among the 184 human Herpesviridae genomic signatures, HSV types 1 or 2 or both were documented in 5.7%, VZV in 39.7%, HHV7 in 38.6%, HHV6 in 15.0%, and Epstein-Barr virus in 0.5%.
Conclusions: This study is the first to identify HSV-positive genomic signatures in clinical AK isolates and/or contact lens cases. Taken together, the high prevalence of HSV genomic signatures in both amebic hosts and lens cases, might signal an unrecognized Acanthamoeba-HSV association and the need for reassessing current management.
Conflict of interest statement
Disclosure:
Figures



Similar articles
-
Evaluation of multiplex real-time polymerase chain reaction for the detection of herpes simplex virus-1 and 2 and varicella-zoster virus in corneal cells from normal subjects and patients with keratitis in India.Indian J Ophthalmol. 2019 Jul;67(7):1040-1046. doi: 10.4103/ijo.IJO_1700_18. Indian J Ophthalmol. 2019. PMID: 31238404 Free PMC article.
-
Acanthamoeba keratitis in noncompliant soft contact lenses users: Genotyping and risk factors, a study from Cairo, Egypt.J Infect Public Health. 2018 May-Jun;11(3):377-383. doi: 10.1016/j.jiph.2017.09.013. Epub 2017 Sep 28. J Infect Public Health. 2018. PMID: 28965795
-
Acanthamoeba keratitis related to contact lens use in a tertiary hospital in China.BMC Ophthalmol. 2019 Sep 18;19(1):202. doi: 10.1186/s12886-019-1210-2. BMC Ophthalmol. 2019. PMID: 31533675 Free PMC article.
-
Twenty years of acanthamoeba diagnostics in Austria.J Eukaryot Microbiol. 2015 Jan-Feb;62(1):3-11. doi: 10.1111/jeu.12149. Epub 2014 Sep 25. J Eukaryot Microbiol. 2015. PMID: 25047131 Free PMC article. Review.
-
Epidemiology of and Genetic Factors Associated with Acanthamoeba Keratitis.Pathogens. 2024 Feb 4;13(2):142. doi: 10.3390/pathogens13020142. Pathogens. 2024. PMID: 38392880 Free PMC article. Review.
References
-
- Bagga B, Joseph J, Garg P, et al. .. Efficacy of topical miltefosine in patients with Acanthamoeba keratitis: a pilot study. Ophthalmology. 2018; 126(5): 768–770. - PubMed
-
- Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol. 2013; 29(4): 181–187. - PubMed
-
- Hammersmith KM. Diagnosis and management of Acanthamoeba keratitis. Curr Opin Ophthalmol. 2006; 17(4): 327–331. - PubMed
-
- Zhang Y, Xu X, Wei Z, Cao K, Zhang Z, Liang Q.. The global epidemiology and clinical diagnosis of Acanthamoeba keratitis. J Infect Public Health. 2023; 16(6): 841–852. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

