Lamp2 Deficiency Enhances Susceptibility to Oxidative Stress-Induced RPE Degeneration
- PMID: 39898910
- PMCID: PMC11798339
- DOI: 10.1167/iovs.66.2.2
Lamp2 Deficiency Enhances Susceptibility to Oxidative Stress-Induced RPE Degeneration
Abstract
Purpose: Autophagy and lysosomal degradation are vital processes that protect cells from oxidative stress. This study investigated the role of lysosome-associated membrane protein 2 (Lamp2), a lysosomal protein essential for autophagosome maturation and lysosome biogenesis, in maintaining retinal health under oxidative stress.
Methods: To induce oxidative stress, young Lamp2 knockout (KO) and wild-type mice received an intravenous injection of a low dose (10 mg/kg) of sodium iodate (NaIO3). We examined retinal histopathology and morphological changes in the RPE. The involvement of resident microglia or infiltrating macrophages was assessed using immunostaining, flow cytometry, and real-time PCR for chemokines and cytokines.
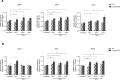
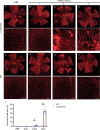
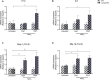
Results: After administering a low-dose NaIO3, Lamp2 KO mice showed significant RPE degeneration, whereas wild-type mice had minimal damage. Histological analysis and electron microscopy revealed significant thinning of the outer nuclear layer and loss of RPE epithelial polarity in Lamp2 KO mice. Additionally, there was a significant increase in ionized calcium-binding adaptor molecule 1-positive microglia and macrophages in the outer retina. Early proliferation of CD45lowMHC-IIlow resident microglia was followed by the infiltration of CD45highLy6Chigh monocytes and the engraftment of CD11b+CD45high monocyte-derived macrophages. Transcript levels of monocyte chemoattractant protein 1, macrophage inflammatory protein 1β, Il- 1β, and Il-6 also increased in the retinas of Lamp2 KO mice. Furthermore, pretreatment with the macrophage-depleting agent clodronate prevented NaIO3-induced RPE degeneration and macrophage infiltration in Lamp2 KO mice.
Conclusions: Lamp2 deficiency, when combined with oxidative stress, leads to RPE degeneration in vivo. Lysosomal dysfunction also promotes macrophage engraftment and triggers neurotoxic inflammation.
Conflict of interest statement
Disclosure:
Figures





Similar articles
-
Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3476-3487. doi: 10.1167/iovs.17-23532. Invest Ophthalmol Vis Sci. 2018. PMID: 30025075
-
Inducible RPE-specific GPX4 knockout causes oxidative stress and retinal degeneration with features of age-related macular degeneration.Exp Eye Res. 2024 Oct;247:110028. doi: 10.1016/j.exer.2024.110028. Epub 2024 Aug 10. Exp Eye Res. 2024. PMID: 39128667
-
Sodium-iodate injection can replicate retinal and choroid degeneration in pigmented mice: Using multimodal imaging and label-free quantitative proteomics analysis.Exp Eye Res. 2024 Oct;247:110050. doi: 10.1016/j.exer.2024.110050. Epub 2024 Aug 14. Exp Eye Res. 2024. PMID: 39151777
-
Loss of DJ-1 elicits retinal abnormalities, visual dysfunction, and increased oxidative stress in mice.Exp Eye Res. 2015 Oct;139:22-36. doi: 10.1016/j.exer.2015.07.014. Epub 2015 Jul 26. Exp Eye Res. 2015. PMID: 26215528 Free PMC article.
-
CAMK2D and Complement Factor I-Involved Calcium/Calmodulin Signaling Modulates Sodium Iodate-Induced Mouse Retinal Degeneration.Invest Ophthalmol Vis Sci. 2025 Jan 2;66(1):63. doi: 10.1167/iovs.66.1.63. Invest Ophthalmol Vis Sci. 2025. PMID: 39873650 Free PMC article.
Cited by
-
Intravenous Sodium Iodate Administration Induces Macula-Specific RPE Damage and Rod-Dominant Apoptosis in the Cynomolgus Monkey.Invest Ophthalmol Vis Sci. 2025 Jul 1;66(9):18. doi: 10.1167/iovs.66.9.18. Invest Ophthalmol Vis Sci. 2025. PMID: 40626808 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

