Are contemporary antifungal doses sufficient for critically ill patients? Outcomes from an international, multicenter pharmacokinetics study for Screening Antifungal Exposure in Intensive Care Units-the SAFE-ICU study
- PMID: 39899034
- PMCID: PMC11903579
- DOI: 10.1007/s00134-025-07793-5
Are contemporary antifungal doses sufficient for critically ill patients? Outcomes from an international, multicenter pharmacokinetics study for Screening Antifungal Exposure in Intensive Care Units-the SAFE-ICU study
Abstract
Purpose: Appropriate antifungal therapy is a major determinant of survival in critically ill patients with invasive fungal disease. We sought to describe whether contemporary dosing of antifungals achieves therapeutic exposures in critically ill patients.
Methods: In a prospective, open-label, multicenter pharmacokinetic study, intensive care unit (ICU) patients prescribed azoles, echinocandins, or polyene antifungals for treatment or prophylaxis of invasive fungal disease were enrolled. Blood samples were collected on two occasions, with three samples taken during a single dosing interval on each occasion. Total concentrations were centrally measured using validated chromatographic methods. Pharmacokinetic parameters were estimated using noncompartmental methods. Antifungal dosing adequacy was assessed using predefined PK/PD targets.
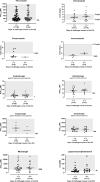
Results: We included 339 patients from 30 ICUs across 12 countries. Median age 62 (interquartile range [IQR], 51-70) years, median APACHE II score 22 (IQR, 17-28), and 61% males. Antifungal therapy was primarily prescribed for treatment (80.8%). Fluconazole was the most frequently prescribed antifungal (40.7%). The most common indication for treatment was intra-abdominal infection (30.7%). Fungi were identified in 45% of patients, of which only 26% had a minimum inhibitory concentration available. Target attainment was higher for patients receiving prophylaxis (> 80% for most drugs). For patients receiving treatment, low target attainment was noted for voriconazole (57.1%), posaconazole (63.2%), micafungin (64.1%) and amphotericin B (41.7%).
Conclusion: This study highlights the varying degrees of target attainment across antifungal agents in critically ill patients. While a significant proportion of patients achieved the predefined PK/PD targets, wide variability and subtherapeutic exposures persist.
Trial registration: ClinicalTrials.gov Identifier: NCT03136926, 2017-04-21.
Keywords: Antifungals; Critically ill; Intensive care unit; Invasive fungal disease; Pharmacokinetics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: JA Roberts has consulted or provided lectures for Qpex, Gilead, Advanz Pharma, Sandoz, Pfizer, MSD, Gilead and Cipla. JJ De Waele has consulted for Biomerieux, Menarini, Monlycke, MSD, Pfizer, Roche Diagnostics, ThermoFisher and Viatris (fees and honoraria paid to institution).
Figures
References
-
- Kullberg BJ, Arendrup MC (2015) Invasive candidiasis. Nat Rev Dis Prim 373:1445–1456. 10.1038/nrdp.2018.27 - PubMed
-
- Thompson GR, Young J-AH (2021) Aspergillus infections. N Engl J Med 385:1496–1509. 10.1056/nejmra2027424 - PubMed
-
- Roberts JA, Kumar A, Lipman J (2017) Right dose, right now: customized drug dosing in the critically ill. Crit Care Med 45:331–336. 10.1097/CCM.0000000000002210 - PubMed
-
- Blot SI, Pea F, Lipman J (2014) The effect of pathophysiology on pharmacokinetics in the critically ill patient - concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. 10.1016/j.addr.2014.07.006 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

