UHRF1 promotes calcium oxalate-induced renal fibrosis by renal lipid deposition via bridging AMPK dephosphorylation
- PMID: 39899077
- PMCID: PMC11790803
- DOI: 10.1007/s10565-025-09991-9
UHRF1 promotes calcium oxalate-induced renal fibrosis by renal lipid deposition via bridging AMPK dephosphorylation
Abstract
Background: Nephrolithiasis, a common urinary system disorder, exhibits high morbidity and recurrence rates, correlating with renal dysfunction and the increased risk of chronic kidney disease. Nonetheless, the precise role of disrupted cellular metabolism in renal injury induced by calcium oxalate (CaOx) crystal deposition is unclear. The purpose of this study is to investigate the involvement of the ubiquitin-like protein containing PHD and RING finger structural domain 1 (UHRF1) in CaOx-induced renal fibrosis and its impacts on cellular lipid metabolism.
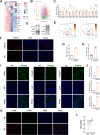
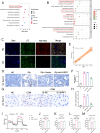
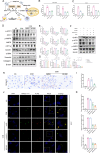
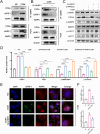
Methods: Various approaches, including snRNA-seq, transcriptome RNA-seq, immunohistochemistry, and western blot analyses, were employed to assess UHRF1 expression in kidneys of nephrolithiasis patients, hyperoxaluric mice, and CaOx-induced renal tubular epithelial cells. Subsequently, knockdown of UHRF1 in mice and cells corroborated its effect of UHRF1 on fibrosis, ectopic lipid deposition (ELD) and fatty acid oxidation (FAO). Rescue experiments using AICAR, ND-630 and Compound-C were performed in UHRF1-knockdown cells to explore the involvement of the AMPK pathway. Then we confirmed the bridging molecule and its regulatory pathway in vitro. Experimental results were finally confirmed using AICAR and chemically modified si-UHRF1 in vivo of hyperoxaluria mice model.
Results: Mechanistically, UHRF1 was found to hinder the activation of the AMPK/ACC1 pathway during CaOx-induced renal fibrosis, which was mitigated by employing AICAR, an AMPK agonist. As a nuclear protein, UHRF1 facilitates nuclear translocation of AMPK and act as a molecular link targeting the protein phosphatase PP2A to dephosphorylate AMPK and inhibit its activity.
Conclusion: This study revealed that UHRF1 promotes CaOx -induced renal fibrosis by enhancing lipid accumulation and suppressing FAO via inhibiting the AMPK pathway. These findings underscore the feasible therapeutic implications of targeting UHRF1 to prevent renal fibrosis due to stones.
Keywords: AMPK; Fatty acid oxidation; Lipid synthesis; Renal fibrosis; UHRF1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Ethics Committee of the Renmin Hospital of Wuhan University approved the clinical specimen collection (approval number: WDRY2021-KS047). The Experimental Animal Welfare and Ethics Committee of Wuhan University People's Hospital (approval number: WDRM20200604) permitted all animal experiments. Consent for publication: Not applicable. Conflicts of interest: The authors declare no competing interests.
Figures



Similar articles
-
CXCR4 inhibition attenuates calcium oxalate crystal deposition-induced renal fibrosis.Int Immunopharmacol. 2022 Jun;107:108677. doi: 10.1016/j.intimp.2022.108677. Epub 2022 Mar 4. Int Immunopharmacol. 2022. PMID: 35255299
-
Nuclear UHRF1 is a gate-keeper of cellular AMPK activity and function.Cell Res. 2022 Jan;32(1):54-71. doi: 10.1038/s41422-021-00565-y. Epub 2021 Sep 24. Cell Res. 2022. PMID: 34561619 Free PMC article.
-
Autophagy inhibition attenuates hyperoxaluria-induced renal tubular oxidative injury and calcium oxalate crystal depositions in the rat kidney.Redox Biol. 2018 Jun;16:414-425. doi: 10.1016/j.redox.2018.03.019. Epub 2018 Apr 2. Redox Biol. 2018. PMID: 29653411 Free PMC article.
-
Kaempferol alleviates calcium oxalate crystal-induced renal injury and crystal deposition via regulation of the AR/NOX2 signaling pathway.Phytomedicine. 2021 Jun;86:153555. doi: 10.1016/j.phymed.2021.153555. Epub 2021 Mar 27. Phytomedicine. 2021. PMID: 33852977
-
Nephrolithiasis: a consequence of renal epithelial cell exposure to oxalate and calcium oxalate crystals.Mol Urol. 2000 Winter;4(4):305-12. Mol Urol. 2000. PMID: 11156696 Review.
References
-
- Alexander RT, Fuster DG, Dimke H. Mechanisms underlying calcium nephrolithiasis. Annu Rev Physiol. 2022;84:559–83. 10.1146/annurev-physiol-052521-121822. - PubMed
-
- Brownsey RW, Zhande R, Boone AN. Isoforms of acetyl-CoA carboxylase: structures, regulatory properties and metabolic functions. Biochem Soc Trans. 1997;25(4):1232–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

