Iron-mediated post-transcriptional regulation in Toxoplasma gondii
- PMID: 39899594
- PMCID: PMC11801735
- DOI: 10.1371/journal.ppat.1012857
Iron-mediated post-transcriptional regulation in Toxoplasma gondii
Abstract
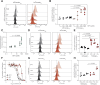
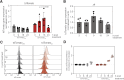
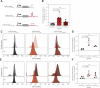
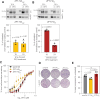
Iron is required to support almost all life; however, levels must be carefully regulated to maintain homeostasis. Although the obligate parasite Toxoplasma gondii requires iron, how it responds upon iron limitation has not been investigated. Here, we show that iron depletion triggers significant transcriptional changes in the parasite, including in iron-dependent pathways. We find that a subset of T. gondii transcripts contain stem-loop structures, which have been associated with post-transcriptional iron-mediated regulation in other cellular systems. We validate one of these (found in the 3' UTR of TGME49_261720) using a reporter cell line. We show that the presence of the stem-loop-containing UTR is sufficient to confer accumulation at the transcript and protein levels under low iron. This response is dose and time-dependent and is specific for iron. The accumulation of transcript is likely driven by an increased reporter mRNA stability under low iron. Interestingly, we find iron-mediated changes in mRNA stability in around 400 genes. To examine the potential mechanism of this stability, we tested aconitase interaction with mRNA in low iron and found 43 enriched transcripts, but no specific interaction with our reporter UTR. However, the endogenous UTR led to maintenance of protein levels and increased survival of the parasite under low iron. Our data demonstrate the existence of iron-mediated post-transcriptional regulation in Toxoplasma for the first time; and suggests iron-mediated regulation may be important to the parasite in low iron environments.
Copyright: © 2025 Sloan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Utilization of inherent miRNAs in functional analyses of Toxoplasma gondii genes.J Microbiol Methods. 2015 Jan;108:92-102. doi: 10.1016/j.mimet.2014.11.014. Epub 2014 Dec 3. J Microbiol Methods. 2015. PMID: 25479428
-
mRNA pseudouridylation affects RNA metabolism in the parasite Toxoplasma gondii.RNA. 2017 Dec;23(12):1834-1849. doi: 10.1261/rna.062794.117. Epub 2017 Aug 29. RNA. 2017. PMID: 28851751 Free PMC article.
-
Evidence for a negative feedback control mediated by the 3' untranslated region assuring the low expression level of the RNA binding protein TcRBP19 in T. cruzi epimastigotes.Biochem Biophys Res Commun. 2013 Jun 28;436(2):295-9. doi: 10.1016/j.bbrc.2013.05.096. Epub 2013 Jun 4. Biochem Biophys Res Commun. 2013. PMID: 23743203
-
mRNA export in the apicomplexan parasite Toxoplasma gondii: emerging divergent components of a crucial pathway.Parasit Vectors. 2018 Jan 25;11(1):62. doi: 10.1186/s13071-018-2648-4. Parasit Vectors. 2018. PMID: 29370868 Free PMC article. Review.
-
Histone mediated gene activation in Toxoplasma gondii.Mol Biochem Parasitol. 2006 Aug;148(2):109-16. doi: 10.1016/j.molbiopara.2006.03.010. Epub 2006 Apr 18. Mol Biochem Parasitol. 2006. PMID: 16644030 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

