Active vision in freely moving marmosets using head-mounted eye tracking
- PMID: 39899712
- PMCID: PMC11831172
- DOI: 10.1073/pnas.2412954122
Active vision in freely moving marmosets using head-mounted eye tracking
Abstract
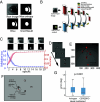
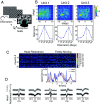
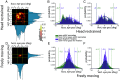
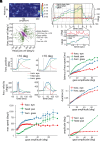
Our understanding of how vision functions as primates actively navigate the real-world is remarkably sparse. As most data have been limited to chaired and typically head-restrained animals, the synergistic interactions of different motor actions/plans inherent to active sensing-e.g., eyes, head, posture, movement, etc.-on visual perception are largely unknown. To address this considerable gap in knowledge, we developed an innovative wireless head-mounted eye-tracking system that performs Chair-free Eye-Recording using Backpack mounted micROcontrollers (CEREBRO) for small mammals, such as marmoset monkeys. Because eye illumination and environment lighting change continuously in natural contexts, we developed a segmentation artificial neural network to perform robust pupil tracking in these conditions. Leveraging this innovative system to investigate active vision, we demonstrate that although freely moving marmosets exhibit frequent compensatory eye movements equivalent to other primates, including humans, the predictability of the visual behavior (gaze) is higher when animals are freely moving relative to when they are head-fixed. Moreover, despite increases in eye/head-motion during locomotion, gaze stabilization remains steady because of an increase in vestibularocular reflex gain during locomotion. These results demonstrate the efficient, dynamic visuo-motor mechanisms and related behaviors that enable stable, high-resolution foveal vision in primates as they explore the natural world.
Keywords: ethology; eye-tracking; gaze stabilization; marmosets; vision.
Conflict of interest statement
Competing interests statement:V.P.S. is inventor on provisional patent application no: US 20230404467 A1 filed by the Regents of the University of California entitled “Head Mounted Camera and Eye Track System for Animals.”
Figures



Update of
-
Active vision in freely moving marmosets using head-mounted eye tracking.bioRxiv [Preprint]. 2024 Nov 21:2024.05.11.593707. doi: 10.1101/2024.05.11.593707. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2412954122. doi: 10.1073/pnas.2412954122. PMID: 38766147 Free PMC article. Updated. Preprint.
Similar articles
-
Active vision in freely moving marmosets using head-mounted eye tracking.bioRxiv [Preprint]. 2024 Nov 21:2024.05.11.593707. doi: 10.1101/2024.05.11.593707. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2412954122. doi: 10.1073/pnas.2412954122. PMID: 38766147 Free PMC article. Updated. Preprint.
-
Dynamics of gaze control during prey capture in freely moving mice.Elife. 2020 Jul 24;9:e57458. doi: 10.7554/eLife.57458. Elife. 2020. PMID: 32706335 Free PMC article.
-
A dynamic sequence of visual processing initiated by gaze shifts.Nat Neurosci. 2023 Dec;26(12):2192-2202. doi: 10.1038/s41593-023-01481-7. Epub 2023 Nov 23. Nat Neurosci. 2023. PMID: 37996524 Free PMC article.
-
The marmoset monkey as a model for visual neuroscience.Neurosci Res. 2015 Apr;93:20-46. doi: 10.1016/j.neures.2015.01.008. Epub 2015 Feb 13. Neurosci Res. 2015. PMID: 25683292 Free PMC article. Review.
-
Eye movements of vertebrates and their relation to eye form and function.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Feb;201(2):195-214. doi: 10.1007/s00359-014-0964-5. Epub 2014 Nov 15. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 25398576 Review.
Cited by
-
Diverse and Flexible Strategies Enable Successful Cooperation in Marmoset Dyads.bioRxiv [Preprint]. 2025 May 7:2025.05.06.652115. doi: 10.1101/2025.05.06.652115. bioRxiv. 2025. PMID: 40654903 Free PMC article. Preprint.
-
Assessing Attentiveness and Cognitive Engagement across Tasks using Video-based Action Understanding in Non-Human Primates.bioRxiv [Preprint]. 2025 Jun 3:2025.05.31.657183. doi: 10.1101/2025.05.31.657183. bioRxiv. 2025. PMID: 40501819 Free PMC article. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

