Pathway Of Low Anterior Resection syndrome (LARS) relief after Surgery (POLARiS): protocol for an international, open-label, multi-arm, phase 3 randomised superiority trial within a cohort, with economic evaluation, process evaluation and qualitative sub-study, to explore the natural history of LARS and compare transanal irrigation and sacral neuromodulation to optimised conservative management for people with major LARS following a high or low anterior resection for colorectal cancer
- PMID: 39900423
- PMCID: PMC11800293
- DOI: 10.1136/bmjopen-2024-092612
Pathway Of Low Anterior Resection syndrome (LARS) relief after Surgery (POLARiS): protocol for an international, open-label, multi-arm, phase 3 randomised superiority trial within a cohort, with economic evaluation, process evaluation and qualitative sub-study, to explore the natural history of LARS and compare transanal irrigation and sacral neuromodulation to optimised conservative management for people with major LARS following a high or low anterior resection for colorectal cancer
Abstract
Introduction: As a result of improving survival rates, the adverse consequences of rectal cancer surgery are becoming increasingly recognised. Low anterior resection syndrome (LARS) is one such consequence and describes a constellation of bowel symptoms after rectal cancer surgery which includes urgency, faecal incontinence, stool clustering and incomplete evacuation. LARS has a significant adverse impact on quality of life (QoL) and symptoms are present in up to 75% of patients in the first year after surgery. Despite this, little is known about the natural history and there is poor evidence to support current treatment options.
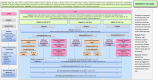
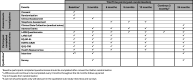
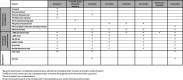
Methods and analysis: The objectives of POLARiS are to explore the natural history of LARS and to evaluate the clinical and cost-effectiveness of transanal irrigation (TAI) or sacral neuromodulation (SNM) compared with optimised conservative management (OCM) for people with major LARS.POLARiS is a prospective, international, open-label, multi-arm, phase 3 randomised superiority trial within a cohort design, with internal pilot phase, qualitative sub-study, process evaluation and economic evaluation. Approximately 1500 adult participants from UK hospitals and 500 from Australian hospitals who have undergone a high or low anterior resection for colorectal cancer in the last 10 years will be recruited into the cohort. Six-hundred participants from the UK and 200 participants from Australia, with major LARS symptoms, defined as a LARS score of ≥30, will be recruited to the randomised controlled trial (RCT) element. Participants entering the RCT will be randomised between OCM, TAI or SNM, all with equal allocation ratios.Cohort and RCT participants will be followed up for a 24-month period, completing a series of questionnaires measuring LARS symptoms and QoL, as well as clinical review for those in the RCT. A process evaluation, qualitative sub-study and economic evaluation will also be conducted.The primary outcome measure of the POLARiS cohort and RCT is the LARS score up to 24 months post-registration/randomisation. Analyses of the RCT will be conducted on an intention-to-treat basis. Comparative effectiveness analyses for each endpoint will consist of two pairwise treatment comparisons: TAI versus OCM and SNM versus OCM. Secondary outcomes include health-related QoL, adverse events, treatment compliance and cost-effectiveness (up to 24 months post-registration/randomisation).
Ethics and dissemination: Ethical approval has been granted by Wales REC 4 (reference: 23/WA/0171) in the UK and Sydney Local Health District HREC (reference: 2023/ETH00749) in Australia. The results of this trial will be disseminated to participants on request and published on completion of the trial in a peer-reviewed journal and at international conferences.
Trial registration number: ISRCTN12834598; ACTRN12623001166662.
Keywords: Clinical Trial; Colorectal surgery; Quality of Life.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: All authors were required to provide ICMJE COI disclosure form, the following disclosures were made: DDS is fully funded through the NIHR as a Research Professor and is an NIHR Efficacy and Mechanism Evaluation Funding Committee member. CHK has carried out speaker and consultancy work for Medtronic and consultancy work for Exero Medical. He is Chief Medical Officer and co-founder of Amber Therapeutics, and CMO and shareholder at Enterika LTD. AQ is a member of ACPGBI Advanced Cancer sub-committee and has received a ZR MedTech Honoraria for lecturing at the Chinese Colorectal Society. JCo has received an educational grant from BD & Medtronic and is Clinical Director of Everywoman Festival. All other authors made no declarations.
Figures


References
-
- Bowel cancer statistics | cancer research UK. [29-Jul-2024]. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... Available. Accessed.
-
- National bowel cancer audit an audit of the care received by people with bowel cancer in England and Wales the national bowel cancer audit (NBOCA) 2023
-
- Survival | bowel cancer | cancer research UK. [30-Jul-2024]. https://www.cancerresearchuk.org/about-cancer/bowel-cancer/survival Available. Accessed.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
