Establishment of a bat lung organoid culture model for studying bat-derived infectious diseases
- PMID: 39900611
- PMCID: PMC11791068
- DOI: 10.1038/s41598-025-88621-0
Establishment of a bat lung organoid culture model for studying bat-derived infectious diseases
Abstract
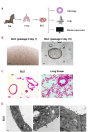
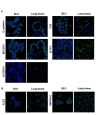
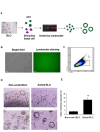
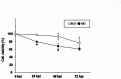
Bat is considered a natural reservoir of various important pathogens, including severe acute respiratory syndrome coronavirus (SARS-CoV), SARS-CoV-2, Ebola virus, and Nipah virus. To study these viruses' pathogenicity and proliferation efficacy and viral tolerance mechanisms in bats, bat-derived cell lines, and primary cultured cells are used. However, these do not adequately reflect the exact biology of bats, and establishing new bat-related research models is necessary. Organoid culture can recapitulate organ structure, functions, and diseases. The respiratory tract is one of the primary routes of viral infection, and the establishment of bat lung organoids (BLO) is necessary to study the viral susceptibility in bats. Therefore, we aimed to establish a culture method of BLO from Rousettus leschenaultia that died of natural causes. The generated BLO successfully recapitulated the characteristics of pulmonary epithelial structure and morphology. BLO expressed the entry receptors for coronavirus, Angiotensin-converting enzyme 2 (ACE2), and Transmembrane Protease Serine 2 (TMPRSS2), and alveolar type 2 cells were successfully sorted from BLO, which has an important role for the development of viral infection in the respiratory system. Furthermore, we showed that BLO had no susceptibility to Pteropine orthoreovirus (PRV) compared to bat intestinal organoids. Collectively, our established bat organoid culture models including this BLO might become promising in vitro biomaterials to study the biology of bat-derived infectious diseases.
Keywords: Rousettus leschenaultii; ACE2; Alveolar type 2 cells; Bat; Lung organoid; TMPRSS2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Tee, K. K. et al. Surveillance, isolation and genomic characterization of Pteropine orthoreovirus of probable bat origin among patients with acute respiratory infection in Malaysia. J. Med. Virol.95, e28520. 10.1002/jmv.28520 (2023). - PubMed
-
- Takemae, H. et al. Isolation of Pteropine Orthoreovirus from Pteropus vampyrus in Garut, Indonesia. Virus Genes. 54, 823–827. 10.1007/s11262-018-1603-y (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

