Three-dimensional mass spectrometry imaging (3D MSI): incorporating top-hat IR-MALDESI and automatic z-axis correction
- PMID: 39900867
- PMCID: PMC11876208
- DOI: 10.1007/s00216-025-05755-w
Three-dimensional mass spectrometry imaging (3D MSI): incorporating top-hat IR-MALDESI and automatic z-axis correction
Abstract
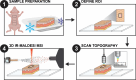
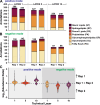
Leveraging a depth profiling approach expands the chemical elucidation of mass spectrometry imaging techniques to another dimension. Three-dimensional MSI (3D MSI) reveals the distribution of analytes with greater anatomical detail to add another level of information in a biological study. Infrared matrix-assisted laser desorption electrospray ionization (IR-MALDESI) has demonstrated utility for an ablation-based approach, enabling simplified sample preparation workflows and streamlined data processing pipelines compared to a serial-sectioning strategy. To improve 3D MSI on the IR-MALDESI platform, two technologies have been characterized in tandem for the intention of minimizing sampling bias: (1) a top-hat optical train and (2) a chromatic confocal probe (CA probe). While the modified optical train creates a square spot size to avoid a Gaussian ablation crater after the analysis of subsequent layers, the CA probe enables automatic z-axis correction (AzC) to maintain the laser's focus on the surface of the sample. The work herein demonstrates the integration and optimization of these technologies on mouse skin, motivated by the clear biological skin layers that result in differential lipid expression and subsequent detection. Results support that a laser energy of 1.3 mJ/burst with the top-hat optical train and a 120 µm step size in the X and Y dimensions presented a comparable depth resolution to previous studies at under 7 µm. Further, the optimized parameters were utilized on two biological replicates to evaluate method reproducibility where lipid annotations and their abundance were considered.
Keywords: 3D; Automatic z-axis correction; Lipids; Mass spectrometry imaging; Top-hat.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: For sample preparation, all aspects of animal care and experimentation in this work were in compliance with NIH guidelines and approved by the NC State University Institutional Animal Care and Use Committee (IACUC, #19–811-B & #23–219). Competing interests: Data analysis for this manuscript was completed in part with MSiReader Pro v2.60 and D.C.M. is a part owner of MSI Software Solutions, LLC.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

