Shift from chronic to episodic migraine frequency in a long-term phase 3 study of galcanezumab
- PMID: 39901101
- PMCID: PMC11792349
- DOI: 10.1186/s10194-025-01956-x
Shift from chronic to episodic migraine frequency in a long-term phase 3 study of galcanezumab
Abstract
Background: Chronic migraine (CM) is a highly disabling form of migraine in which patients have ≥ 15 headache days per month, of which at least 8 have the features of migraine. Galcanezumab is a monoclonal antibody to calcitonin gene-related peptide which is approved for the preventive treatment of migraine. Ability to convert patients from chronic migraine frequency to episodic migraine (EM) frequency is a clinically relevant and desirable outcome when prescribing preventive treatments to patients with CM.
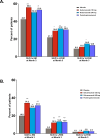
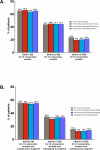
Methods: Patients aged 18-65 years with an ICHD-3β diagnosis of CM were randomized 2:1:1 to receive monthly injections of placebo (N = 558), galcanezumab 120 mg with a 240-mg loading dose (N = 278), or galcanezumab 240 mg (N = 277) during a 3-month double-blind period of the phase 3 REGAIN trial. Patients could subsequently enter a 9-month open-label extension in which they received galcanezumab 120 mg or 240 mg/month per investigator's discretion. In this post-hoc analysis, we assessed the percentages of patients who shifted to EM (< 8 migraine headache days or < 15 headache days/month), low frequency EM (LFEM; <8 migraine headache days/month), and very low frequency EM (VLFEM; <4 migraine headache days/month) for at least 3 consecutive months. Double-blind percentage comparisons versus placebo represent modeled estimates from raw rates.
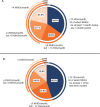
Results: At baseline, patients had a mean of 19.4 migraine headache days per month (SD = 4.5) and 21.4 headache days per month (SD = 4.1). During the 3-month double-blind treatment period, a greater percentage of galcanezumab-treated patients shifted to EM frequency and maintained it across all 3 months (31.5%) than did placebo-treated patients (19.8%, p < 0.001). Among galcanezumab-treated patients across the entire 12-month trial, 65.1% shifted from CM to EM frequency, with 44.2% shifting to LFEM and 21.5% shifting to VLFEM for ≥ 3 consecutive months. Proportions of patients shifting from CM to EM frequency for ≥ 3 consecutive months and until last patient visit were: 55.0% to EM; 33.4% to LFEM; 13.9% to VLFEM.
Conclusion: These results suggest that galcanezumab helped a majority of patients convert from chronic to episodic migraine frequency over the course of this 12-month study.
Trial registration: Clinicaltrials.gov NCT02614261, first registered November 25, 2015.
Keywords: Calcitonin gene-related peptide; Chronic migraine; Episodic migraine; Galcanezumab; Migraine prevention.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: H-CD received honoraria for contribution to advisory boards or oral presentations from: Lilly, Lundbeck, Novartis, Pfizer and Teva. The German Research Council (DFG) supports his headache research, and serves on the editorial boards of Cephalalgia, Lancet Neurology and Drugs. HCD and KAD are employees of Eli Lilly and Company. N.A.H is a speaker and consultant for Impel, Eli Lilly and Company, and AbbVie. SKA is a former employee of Eli Lilly and is currently employed by C2N diagnostics. SL is an employee of Syneos Health that was contracted by Eli Lilly and Company for this work.
Figures
References
-
- Schwedt TJ (2014) Chronic migraine. BMJ 348:g1416 - PubMed
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The International classification of Headache disorders, 3rd edition. Cephalalgia 38(1):1–211 - PubMed
-
- Buse DC, Manack A, Serrano D, Turkel C, Lipton RB (2010) Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry 81(4):428–432 - PubMed
-
- Lanteri-Minet M (2014) Economic burden and costs of chronic migraine. Curr Pain Headache Rep 18(1):385 - PubMed
-
- Blumenfeld AM, Bloudek LM, Becker WJ, Buse DC, Varon SF, Maglinte GA et al (2013) Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache 53(4):644–655 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

