O-GlcNAcylation attenuates ischemia-reperfusion-induced pulmonary epithelial cell ferroptosis via the Nrf2/G6PDH pathway
- PMID: 39901237
- PMCID: PMC11792224
- DOI: 10.1186/s12915-025-02126-w
O-GlcNAcylation attenuates ischemia-reperfusion-induced pulmonary epithelial cell ferroptosis via the Nrf2/G6PDH pathway
Abstract
Background: Lung ischemia-reperfusion (I/R) injury is a common clinical pathology associated with high mortality. The pathophysiology of lung I/R injury involves ferroptosis and elevated protein O-GlcNAcylation levels, while the effect of O-GlcNAcylation on lung I/R injury remains unclear. This research aimed to explore the effect of O-GlcNAcylation on reducing ferroptosis in pulmonary epithelial cells caused by I/R.
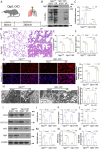
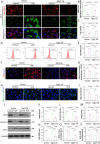
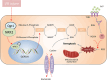
Results: First, we identified O-GlcNAc transferase 1 (Ogt1) as a differentially expressed gene in lung epithelial cells of acute lung injury/acute respiratory distress syndrome (ALI/ARDS) patients, using single-cell sequencing, and Gene Ontology analysis (GO analysis) revealed the enrichment of the ferroptosis process. We found a time-dependent dynamic alteration in lung O-GlcNAcylation during I/R injury. Proteomics analysis identified the differentially expressed proteins enriched in ferroptosis and multiple redox-related pathways based on KEGG annotation. Thus, we generated Ogt1-conditional knockout mice and found that Ogt1 deficiency aggravated ferroptosis, as evidenced by lipid reactive oxygen species (lipid ROS), malondialdehyde (MDA), Fe2+, as well as alterations in critical protein expression glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11). Consistently, we found that elevated O-GlcNAcylation inhibited ferroptosis sensitivity in hypoxia/reoxygenation (H/R) injury-induced TC-1 cells via O-GlcNAcylated NF-E2-related factor-2 (Nrf2). Furthermore, both the chromatin immunoprecipitation (ChIP) assay and the dual-luciferase reporter assay indicated that Nrf2 could bind with translation start site (TSS) of glucose-6-phosphate dehydrogenase (G6PDH) and promote its transcriptional activity. As an important rate-limiting enzyme in the pentose phosphate pathway (PPP), elevated G6PDH provided a mass of nicotinamide adenine dinucleotide phosphate (NADPH) to improve the redox state of glutathione (GSH) and eventually led to ferroptosis resistance. Rescue experiments proved that Nrf2 knockdown or Nrf2-T334A (O-GlcNAcylation site) mutation abolished the protective effect of ferroptosis resistance.
Conclusions: In summary, we revealed that O-GlcNAcylation could protect against I/R lung injury by reducing ferroptosis sensitivity via the Nrf2/G6PDH pathway. Our work will provide a new basis for clinical therapeutic strategies for pulmonary ischemia-reperfusion-induced acute lung injury.
Keywords: Ferroptosis; G6PDH; Lung ischemia–reperfusion; Nrf2; O-GlcNAcylation; Ogt1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The procedures were conducted in accordance with the principles of Animal Care of Wuhan University (Wuhan, China) and approved by the Laboratory Animal Committee (ZN2022284). Ethical principles for clinical research of Zhongnan Hospital of Wuhan University were followed, with approval from the Medical Ethics Committee (2023138 K). All participants signed informed consent forms, and strict measures were taken to protect their privacy and data security. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures







References
-
- Yabuki H, Watanabe T, Oishi H, Katahira M, Kanehira M, Okada Y: Muse Cells and Ischemia-Reperfusion Lung Injury. In: Muse Cells: Endogenous Reparative Pluripotent Stem Cells. Edited by Dezawa M, vol. 1103. Cham: Springer International Publishing Ag; 2018: 293–303. - PubMed
-
- Li C, Sun G, Chen B, Xu L, Ye Y, He J, Bao Z, Zhao P, Miao Z, Zhao L, et al. Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res. 2021;174: 105933. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

