Healthcare Professionals' Knowledge, Views, and Perceptions of the Roles and Functions of Research Ethics Committees: A Web-Based Cross-Sectional Survey
- PMID: 39901523
- PMCID: PMC11790396
- DOI: 10.3346/jkms.2025.40.e9
Healthcare Professionals' Knowledge, Views, and Perceptions of the Roles and Functions of Research Ethics Committees: A Web-Based Cross-Sectional Survey
Abstract
Background: This survey examined healthcare professionals' knowledge, views, and perceptions of the responsibilities and functions of Research Ethics Committees (RECs). The study aimed to analyze ethical principles and operational issues faced by RECs and guide researchers, journal editors, and publishers on publication ethics notes.
Methods: A cross-sectional survey was conducted using the SurveyMonkey.com platform to assess healthcare professionals' knowledge, views, and practices concerning RECs' responsibilities, functions, and roles. The survey focused on REC definitions, functions, research types that require REC approval, and research protocols' evaluation time frames. It also reflected on ethics considerations and REC adaptations during the coronavirus disease 2019 (COVID-19) pandemic, REC member qualifications, evaluation periods, and additional challenges confronting RECs. Convenience sampling was adopted, and the survey was distributed via social media platforms.
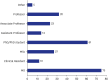
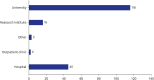
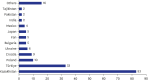
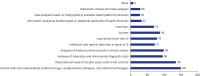
Results: The survey was based on an analysis of questionnaires filled by 182 responders (104 females [57.1%] and 76 males [41.8%]), with a median age of 36. The survey respondents were from 28 different countries. The top three countries with most responders were Kazakhstan (n = 83), Türkiye (n = 33) and Poland (n = 10). Most participants (n = 128, 70.3%) were familiar with the definition of RECs and recognized the importance of REC approval for clinical trials and interventional research. Research study protocols should be submitted for REC evaluation and approval during the planning phase, according to 145 responders (79.7%). Participants emphasized the significance of formal ethics training for REC members. The involvement in research approved by RECs was also viewed as an important precondition for membering RECs. Participants suggested online submissions (n = 127, 69.8%), virtual meetings (n = 99, 54.4%), and fast evaluation schedules for low-risk research protocols (n = 77, 42.3%) during the COVID-19 pandemic.
Conclusion: Healthcare professionals comprehend the basics of REC duties and responsibilities. However, improvements in the consistency and efficiency of ethics evaluations are still warranted. The COVID-19 pandemic stressed the importance of adaptive REC procedures; researchers, editors, and publishers learned a vitally important lesson. More efforts are warranted to increase REC member training, simplify administrative procedures, and define standard operating procedures in times of crisis. Continuous progress in these areas will allow RECs to maintain high ethical standards while supporting productive research. Editors and publishers will greatly benefit from related advances in research ethics considerations.
Keywords: Editorial Policies; Health Personnel; Periodicals as Topic; Publication Ethics; Publishing; Research Ethics Committees; Surveys and Questionnaires.
© 2025 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures



Similar articles
-
The readiness of the Asian research ethics committees in responding to the COVID-19 pandemic: A multi-country survey.F1000Res. 2024 Jan 8;13:19. doi: 10.12688/f1000research.143138.1. eCollection 2024. F1000Res. 2024. PMID: 39165349 Free PMC article.
-
Surveying the Indian research ethics committee response to the COVID-19 pandemic.Dev World Bioeth. 2024 Sep;24(3):243-253. doi: 10.1111/dewb.12417. Epub 2023 Aug 4. Dev World Bioeth. 2024. PMID: 37540074
-
Identifying structures, processes, resources and needs of research ethics committees in Egypt.BMC Med Ethics. 2010 Jun 28;11:12. doi: 10.1186/1472-6939-11-12. BMC Med Ethics. 2010. PMID: 20584332 Free PMC article.
-
Adherence with reporting of ethical standards in COVID-19 human studies: a rapid review.BMC Med Ethics. 2021 Jun 28;22(1):80. doi: 10.1186/s12910-021-00649-9. BMC Med Ethics. 2021. PMID: 34182962 Free PMC article.
-
Are Research Ethics Committees Prepared for Community-Based Participatory Research?J Empir Res Hum Res Ethics. 2015 Dec;10(5):488-95. doi: 10.1177/1556264615615008. Epub 2015 Nov 1. J Empir Res Hum Res Ethics. 2015. PMID: 26527370 Review.
Cited by
-
Perspectives of Artificial Intelligence Use for In-House Ethics Checks of Journal Submissions.J Korean Med Sci. 2025 Jun 2;40(21):e170. doi: 10.3346/jkms.2025.40.e170. J Korean Med Sci. 2025. PMID: 40461144 Free PMC article. Review.
References
-
- Rotolo D, Camerani R, Grassano N, Martin BR. Why do firms publish? A systematic literature review and a conceptual framework. Res Policy. 2022;51(10):104606
-
- Brown C, Spiro J, Quinton S. The role of research ethics committees: friend or foe in educational research? An exploratory study. Br Educ Res J. 2020;46(4):747–769.
-
- Scheibner J, Ienca M, Kechagia S, Troncoso-Pastoriza JR, Raisaro JL, Hubaux JP, et al. Data protection and ethics requirements for multisite research with health data: a comparative examination of legislative governance frameworks and the role of data protection technologies. J Law Biosci. 2020;7(1):lsaa010. - PMC - PubMed
-
- Rothstein MA, Zawati MH, Thorogood A, Beauvais MJS, Joly Y, Brothers KB, et al. Streamlining ethics review for international health research. Science. 2022;375(6583):825–826. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

