p16/ki-67 dual stain triage of individuals positive for HPV to detect cervical precancerous lesions
- PMID: 39901857
- PMCID: PMC12008826
- DOI: 10.1002/ijc.35353
p16/ki-67 dual stain triage of individuals positive for HPV to detect cervical precancerous lesions
Abstract
p16/Ki-67 dual stain is a biomarker-based test that can identify oncogenic transformation in cervical cells with higher sensitivity than cervical cytology, using the same samples taken for human papillomavirus (HPV) testing and liquid-based cytology. Dual stain is approved by the US Food and Drug Administration (FDA) for triage of women with positive results by primary HPV testing or by HPV/cytology co-testing and has recently been incorporated into management guidelines. In this review, we summarize the data showing the utility of dual stain in detecting precancers, reducing the number of unnecessary colposcopies, and reassuring women who test negative. We also discuss the implications of dual stain for future treatment practice and health economics.
Keywords: HPV; cervical cancer screening; dual stain; triage.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
T. P. has been a speaker and consultant to Roche Diagnostics. R. R. is an employee of Roche Diagnostics and reports minority stock ownership in Roche Holding AG. The other authors have no conflicts to declare.
Figures

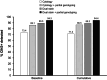
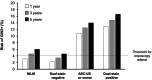
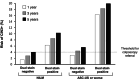
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- Lăără E, Day NE, Hakama M. Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet. 1987;1(8544):1247‐1249. - PubMed
-
- Jansen EE, Zielonke N, Gini A, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer. 2020;127:207‐223. - PubMed
-
- Mayrand MH, Duarte‐Franco E, Rodrigues I, et al. Human papillomavirus DNA versus papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579‐1588. - PubMed

