Dynamic evolution of TCF3-PBX1 leukemias at the single-cell level under chemotherapy pressure
- PMID: 39901941
- PMCID: PMC11788586
- DOI: 10.1002/hem3.70071
Dynamic evolution of TCF3-PBX1 leukemias at the single-cell level under chemotherapy pressure
Abstract
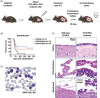
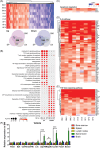
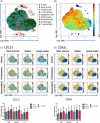
Acute lymphoblastic leukemia (ALL) is the most common childhood cancer. The translocation t(1;19), encoding the TCF3-PBX1 fusion, is associated with intermediate risk and central nervous system (CNS) infiltration at relapse. Using our previously generated TCF3-PBX1 conditional knock-in mice, we established a model to study relapsed clones after in vivo chemotherapy treatment, CNS infiltration, and clonal dynamic evolution of phenotypic diversity at the single cell-level using next-generation sequencing technologies and mass cytometry. Mice transplanted with TCF3-PBX1 + leukemia cells and treated with vehicle succumbed to disease, whereas 40% of treated mice with prednisolone or daunorubicin survived. Bulk and single-cell RNA sequencing of FACS-sorted GFP+ cells from TCF3-PBX1 + leukemias arising after chemotherapy treatment revealed that apoptosis, interleukin-, and TGFβ-signaling pathways were regulated in CNS-infiltrating leukemic cells. Across tissues, upregulation of the MYC signaling pathway was detected in persisting leukemic cells and its downregulation by BRD3/4 inhibition increased sensitivity to chemotherapy. In TCF3-PBX1+ leukemia cells collected after chemotherapy treatment, mass cytometry identified increased phosphorylation of STAT3/5 upon preBCR stimulation, which was susceptible to inhibition by the proteasome inhibitor bortezomib. In summary, we developed a TCF3-PBX1+ ALL mouse model and characterized relapsed disease after in vivo chemotherapy and cell phenotype dependence on microenvironment. Transcriptomics and phospho-proteomics revealed distinct pathways that may underlie chemotherapy resistance and might be suitable for pharmacological interventions in human ALL.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare no conflicting financial interest with the submission of this article. Jesús Duque‐Afonso received speakers honoraria from Roche, Amgen, Riemser, SOBI, IPSEN, Abbvie, Beigene, NovoNordisk, and AstraZeneca and travel support from Lilly, Roche, Gilead, IPSEN, SOBI, and Beigene.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
