Incremental Modification in the Existing Approaches for Affinity Chromatographic Enrichment of Phosphoproteins Improves Their Profile in Liquid Chromatography-Tandem Mass Spectrometry Analysis
- PMID: 39902464
- PMCID: PMC11789765
- DOI: 10.1002/ansa.202400058
Incremental Modification in the Existing Approaches for Affinity Chromatographic Enrichment of Phosphoproteins Improves Their Profile in Liquid Chromatography-Tandem Mass Spectrometry Analysis
Abstract
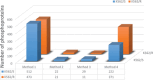
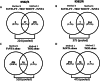
Cell signalling is a vital process in cell physiology, which is driven by protein phosphorylation. Global phosphoproteome analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS) has thus gained importance in cell signalling research. However, phosphoprotein identification by LC-MS/MS in whole cell lysates, which are complex protein mixtures, is hindered by their poor ionization coupled with suppression of peaks due to low abundance. Enrichment by immobilized metal ion- and metal oxide-affinity chromatography (IMAC and MOAC), which preferentially enrich multi- and mono-phosphorylated proteins, respectively, have improved their detection by MS. However, preferential enrichment limits phosphoproteome coverage in global analyses of cell lysates which contain mono- and multi-phosphorylated proteins. Improvement in their coverage by sequential elution approach that exploits the complementary chemistries of these matrices has been reported. In this study, we observed that the number of phosphoproteins detected using the sequential elution approach was lower (∼250-400) as compared to the theoretically predicted number (>500) based on their reported 30% abundance in the cell proteome (1700-2200 proteins detected by MS in our cell lines). Acknowledging the merit of using multiple matrices, we used IMAC and MOAC individually and pooled the data. We observed a remarkable increase (>30%) in phosphoproteome coverage. Further, though 98% of phosphoproteins were enriched by IMAC, among the remaining 2%, those detected exclusively by MOAC were biologically important. This justified the use of multiple matrices. Thus, an incremental modification of using multiple matrices individually rather than sequentially and pooling the data markedly improved the phosphoproteome coverage, which can positively impact cell signalling research.
Keywords: affinity chromatography; enrichment; phosphoproteins.
© 2024 The Author(s). Analytical Science Advances published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Graves J. D. and Krebs E. G., “Protein Phosphorylation and Signal Transduction,” Pharmacology and Therapeutics 82, no. 2–3, (1999): 111–121. - PubMed
-
- Cohen P., “The Role of Protein Phosphorylation in Human Health and Disease. The Sir Hans Krebs Medal Lecture,” European Journal of Biochemistry 268, no. 19, (2001): 5001–5010. - PubMed
-
- Yu L. R., Issaq H. J., and Veenstra T. D., “Phosphoproteomics for the Discovery of Kinases as Cancer Biomarkers and Drug Targets,” Proteomics‐Clinical Applications 1, no. 9, (2007): 1042–1057. - PubMed
LinkOut - more resources
Full Text Sources
