Adaptable, wearable, and stretchable coils: A review
- PMID: 39902582
- PMCID: PMC11893040
- DOI: 10.1002/mrm.30428
Adaptable, wearable, and stretchable coils: A review
Abstract
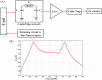
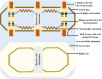
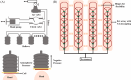
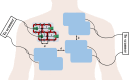
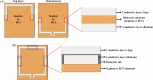
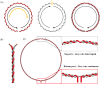
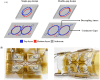
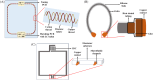
Over the last four decades, there have been various evolutions in the design and development of coils, from volume coils to the recent introduction of wireless receive arrays. A recent aim has been to develop coils that can closely conform to the anatomy of interest to increase the acquired signal. This goal has given rise to designs ranging from adaptable transmit coils to on-body stretchable receive arrays made using fabric or elastomer substrates. This review covers the design, fabrication details, experimental setup, and MRI results of adaptable, wearable, and stretchable MRI coils. The active and passive automatic tuning and matching strategies are examined with respect to mitigating signal-to-noise ratio reduction when the coil form is altered. A brief discussion of wireless MRI coils, which provide a solution to overcome the cabling issues associated with MRI coil development, is also included. The adaptable, wearable, and stretchable coils and various coil tuning techniques represent innovative radiofrequency coil solutions that pave the way for next-generation MRI hardware development.
Keywords: RF coils; adaptable; automatic tuning; stretchable; wearable; wireless MRI.
© 2025 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

