Performance of Three Continuous Glucose Monitoring Systems in Adults With Type 1 Diabetes
- PMID: 39902649
- PMCID: PMC11795573
- DOI: 10.1177/19322968251315459
Performance of Three Continuous Glucose Monitoring Systems in Adults With Type 1 Diabetes
Abstract
Background: The performance of continuous glucose monitoring (CGM) systems is difficult to compare due to different study designs and a lack of head-to-head studies. This study evaluated the performance of FreeStyle Libre 3 (FL3), Dexcom G7 (DG7), and Medtronic Simplera (MSP) against different comparator methods and during clinically relevant glycemic scenarios.
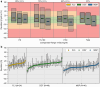
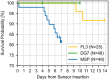
Method: Twenty-four adult participants with type 1 diabetes mellitus wore one sensor of each CGM system in parallel for up to 15 days. Sensors of DG7 and MSP were exchanged on days 5 and 8, respectively. Three 7-hour sessions with 15-minute comparator blood glucose-level measurements using YSI 2300 (YSI, venous), Cobas Integra (INT, venous), and Contour Next (CNX, capillary) were conducted on days 2, 5, and 15. Simultaneously, glucose-level excursions with transient hyperglycemia and hypoglycemia were induced according to a recently published testing procedure. The accuracy was evaluated using various metrics, including mean absolute relative differences (MARDs).
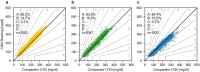
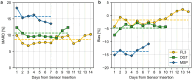
Results: Compared with YSI data, the MARDs of FL3, DG7, and MSP were 11.6%, 12.0%, and 11.6%, respectively. Relative to the INT data, the corresponding MARDs were 9.5%, 9.9%, and 13.9%, respectively, and compared with CNX data, MARDs were 9.7%, 10.1%, and 16.6%, respectively. Both FL3 and DG7 showed better accuracy in the normoglycemic and hyperglycemic range, while MSP performed better in the hypoglycemic range.
Conclusions: Performance results of all CGM systems varied depending on the comparator method. However, across comparators FL3 and DG7 tended to be more accurate compared with MSP. All CGM systems showed a lower accuracy compared with previous studies, emphasizing the need for comprehensive study design guidelines.
Keywords: CG-DIVA; CGM performance; accuracy; comparator method; dynamic glucose regions.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.F. is the general manager and medical director of the Institute for Diabetes Technology (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies, for example, with medical devices for diabetes therapy on its own initiative and on behalf of various companies. G.F./IfDT have received research support, speakers’ honoraria, or consulting fees in the last three years from Abbott, Ascensia, Berlin Chemie, Boydsense, Dexcom, Lilly Deutschland, Novo Nordisk, Perfood, Pharmasens, Roche, Sinocare, Terumo, and Ypsomed. M.E., D.W., S.W., S.P., M.L., N.J., S.Ö., and C.H. are employees of IfDT. M.S., V.B.M., R.S., and D.B. have no conflict of interest.
Figures


References
-
- Freckmann G, Pleus S, Schauer S, et al.. Choice of continuous glucose monitoring systems may affect metrics: clinically relevant differences in times in ranges. Exp Clin Endocrinol Diabetes. 2022;130(5):343-350. - PubMed
-
- Yeoh E, Png D, Khoo J, et al.. A head-to-head comparison between Guardian Connect and FreeStyle Libre systems and an evaluation of user acceptability of sensors in patients with type 1 diabetes. Diabetes Metab Res Rev. 2022;38(7):e3560. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

