Spatial propagation of temperate phages within and among biofilms
- PMID: 39903123
- PMCID: PMC11831127
- DOI: 10.1073/pnas.2417058122
Spatial propagation of temperate phages within and among biofilms
Abstract
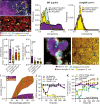
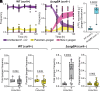
Bacteria form groups composed of cells and a secreted polymeric matrix that controls their spatial organization. These groups-termed biofilms-can act as refuges from environmental disturbances and from biotic threats, including phages. Despite the ubiquity of temperate phages and bacterial biofilms, live propagation of temperate phages within biofilms has not been characterized on cellular spatial scales. Here, we leverage several approaches to track temperate phages and distinguish between lytic and lysogenic host infections. We determine that lysogeny within Escherichia coli biofilms initially occurs within a predictable region of cell group packing architecture on the biofilm periphery. Because lysogens are generally found on the periphery of large cell groups, where lytic viral infections also reduce local biofilm structural integrity, lysogens are predisposed to disperse into the passing liquid and are overrepresented in downstream biofilms formed from the dispersal pool of the original biofilm-phage system. Comparing our results with those for virulent phages reveals that temperate phages have unique advantages in propagating over long spatial and time scales within and among bacterial biofilms.
Keywords: biofilm; dispersal; matrix; phage; spatial ecology.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


Update of
-
Spatial propagation of temperate phages within and among biofilms.bioRxiv [Preprint]. 2024 Jul 23:2023.12.20.571119. doi: 10.1101/2023.12.20.571119. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2417058122. doi: 10.1073/pnas.2417058122. PMID: 38187755 Free PMC article. Updated. Preprint.
References
-
- Krause J., et al. , Living in Groups (OUP Oxford, 2002).
-
- Couzin I. D., Krause J., James R., Ruxton G. D., Franks N. R., Collective memory and spatial sorting in animal groups. J. Theor. Biol. 218, 1–11 (2002). - PubMed
-
- Roussi A., Why gigantic locust swarms are challenging governments and researchers. Nature 579, 330 (2020). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

