CD19.CAR T-cell-derived extracellular vesicles express CAR and kill leukemic cells, contributing to antineoplastic therapy
- PMID: 39903124
- PMCID: PMC12180994
- DOI: 10.1182/bloodadvances.2024014860
CD19.CAR T-cell-derived extracellular vesicles express CAR and kill leukemic cells, contributing to antineoplastic therapy
Abstract
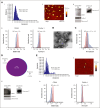
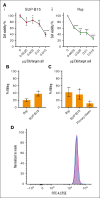
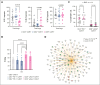
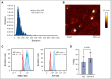
Chimeric antigen receptor (CAR) T-cell-derived extracellular vesicles (EVs) might represent a new therapeutic tool for boosting CAR T-cell antileukemic effects. Here, a cohort of 22 patients who received infusion with CD19 CAR T cells were monitored for the presence of circulating CD19 CAR+ T-cell-derived EVs (CD19.CAR+EVs), which were then separated and functionally characterized for their killing abilities. A good manufacturing practice (GMP)-compliant separation method was also developed. Results demonstrated that CD19.CAR+EVs were detectable in peripheral blood up to 2 years after infusion, indicating long-lasting persistence of their parental cells. Notably, early decreases of circulating CD19.CAR+EV concentrations correlated with failure of CAR T-cell therapy. Circulating CD19.CAR+EVs displayed a median size (standard deviation) of 133.1 ± 65.5 nm and carried a proapoptotic protein cargo. These EVs expressed higher CAR levels than their parental cells. Furthermore, CD19.CAR+EVs did not activate heterologous T cells and produced significant, specific, and dose-dependent cytotoxic effects on CD19+ cell lines and primary cells. The new GMP-compliant EV isolation method allowed for a recovery of 63% ± 5.7% of CD19.CAR+EVs. A deeper analysis of the different protein cargoes carried by EVs derived from different CAR T-cell subpopulations identified a proapoptotic functional pathway linked to CD8+LAG-3+ EVs. Overall, our data indicate that CD19.CAR+EVs may be proposed as promising dynamic new biomarkers of CAR T-cell activity and, by contributing to the direct killing of leukemic targets, represent a new product with strong therapeutic potential that could be infused independently of CAR T cells.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures



Comment in
-
Extracellular vesicles for CAR T-cell therapy immunomonitoring.Blood Adv. 2025 Jun 24;9(12):2920-2921. doi: 10.1182/bloodadvances.2025015988. Blood Adv. 2025. PMID: 40504542 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

