Pulmonary vascular remodeling in Fra-2 transgenic mice is driven by type 2 inflammation and accompanied by pulmonary vascular hyperresponsiveness
- PMID: 39903186
- PMCID: PMC7617946
- DOI: 10.1152/ajplung.00274.2024
Pulmonary vascular remodeling in Fra-2 transgenic mice is driven by type 2 inflammation and accompanied by pulmonary vascular hyperresponsiveness
Abstract
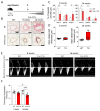
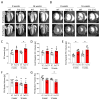
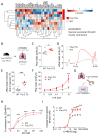
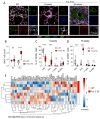
Lung vessel remodeling leads to increased pulmonary vascular resistance, causing pulmonary arterial hypertension (PAH), and consequently right ventricular hypertrophy and failure. In patients suffering from systemic sclerosis (SSc), PAH can occur and is a life-threatening complication. Dysregulation of immune processes plays a crucial role in pulmonary vascular remodeling, as has previously been shown in Fos-related antigen-2 (Fra-2) transgenic (TG) mice, a model of SSc-PAH. Here, we investigate whether vascular remodeling in the Fra-2 TG model is driven by type 2 inflammation and is associated with vascular hyperresponsiveness, an important feature of PAH pathobiology. Basal pulmonary arterial pressure and pulmonary vascular responsiveness to hypoxic ventilation and serotonin were increased in isolated, perfused, and ventilated lungs of Fra-2 TG mice compared with wild-type (WT) littermates. Similarly, contractile responses of isolated intrapulmonary arteries were elevated in Fra-2 TG mice. We also observed increased expression of contractile genes in Fra-2 overexpressing human pulmonary arterial smooth muscle cells (PASMCs) with elevated intracellular calcium levels after interleukin (IL)-13 stimulation. These findings were corroborated by transcriptomic data highlighting dysregulation of vascular smooth muscle cell contraction and type 2 inflammation in Fra-2 TG mice. In vivo, type 2-specific anti-inflammatory treatment with IL-13 neutralizing antibodies improved vascular remodeling in Fra-2 TG mice, similar to corticosteroid treatment with budesonide. Our results underscore the importance of type 2 inflammation and its potential therapeutic value in PAH-associated pulmonary vascular remodeling and hyperresponsiveness in SSc-PAH.NEW & NOTEWORTHY In patients suffering from systemic sclerosis (SSc), pulmonary arterial hypertension (PAH) is a life-threatening complication linked to immune dysregulation. Preclinical analyses in Fos-related antigen-2 (Fra-2) transgenic (TG) mice, a model of SSc-PAH, identify type 2 inflammation as a key driver of vascular remodeling. Anti-inflammatory treatment targeting type 2 inflammation via IL-13 neutralizing antibodies improved pulmonary vascular remodeling. Thus, type 2-specific anti-inflammatory treatment may be a promising therapeutic approach in SSc-PAH.
Keywords: hypoxic pulmonary vasoconstriction; pulmonary hypertension; type 2 inflammation; vascular hyperresponsiveness; vascular remodeling.
Conflict of interest statement
CT received funding for research from Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin e. V., Bayer HealthCare, Boehringer Ingelheim, and for lectures and advisory from Actelion Pharmaceuticals, AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, GlaxoSmithKline, and for non-financial support from Actelion, ALK-Abelló, Bayer HealthCare, Boehringer Ingelheim and GlaxoSmithKline.
Figures

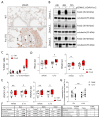
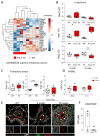
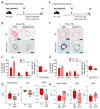
Comment in
-
Targeting type-2 inflammation in pulmonary hypertension: lessons from a novel model.Am J Physiol Lung Cell Mol Physiol. 2025 May 1;328(5):L669-L670. doi: 10.1152/ajplung.00109.2025. Epub 2025 Apr 7. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40192583 Free PMC article. No abstract available.
References
-
- Kumar R, Mickael C, Chabon J, Gebreab L, Rutebemberwa A, Garcia AR, Koyanagi DE, Sanders L, Gandjeva A, Kearns MT, Barthel L, et al. The Causal Role of IL-4 and IL-13 in Schistosoma mansoni Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine. 2015;192:998–1008. doi: 10.1164/rccm.201410-1820OC. - DOI - PMC - PubMed
-
- Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, Tiede H, et al. Vascular Receptor Autoantibodies in Pulmonary Arterial Hypertension Associated with Systemic Sclerosis. Am J Respir Crit Care Med. 2014;190:808–817. - PubMed
-
- Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, Grunig E, et al. Pulmonary arterial remodeling induced by a Th2 immune response. The Journal of Experimental Medicine. 2008;205:361–372. doi: 10.1084/jem.20071008. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

