Regulation by Trace Amine-Associated Receptor 1 (TAAR1) of Dopaminergic-GABAergic Interaction in the Striatum: Effects of the Enhancer Drug (-)BPAP
- PMID: 39903411
- PMCID: PMC11794408
- DOI: 10.1007/s11064-025-04337-7
Regulation by Trace Amine-Associated Receptor 1 (TAAR1) of Dopaminergic-GABAergic Interaction in the Striatum: Effects of the Enhancer Drug (-)BPAP
Abstract
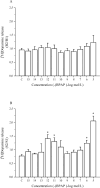
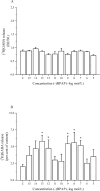
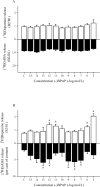
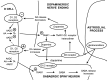
Although it is well documented that the striatal GABAergic projection neurons receive excitatory and inhibitory dopaminergic innervation via D1 and D2 receptors, the trace amine-associated receptor 1 (TAAR1)-mediated regulation of this neural connection is much less studied. The presence of TAAR1 was originally detected in brain aminergic neurons, with recent evidence indicating its presence in striatal GABAergic neurons as well. The objective of the present study was to demonstrate the role of TAAR1 and signaling in dopaminergic-GABAergic interaction in the neural circuitry of the striatum. Besides trace amines, which are considered natural ligands for TAAR1, series of different exogenous drugs were identified to act on this receptor. Using the dopaminergic activity enhancer compound (-)BPAP ((-)-1-(benzofuran-2-yl)-2-propylaminopentane HCl), a potential agonist for TAAR1, we have found that it increased the electrical stimulation-induced [3H]dopamine release in rat striatal slices. This effect of (-)BPAP occurred parallel with increases of [3H]GABA release in striatum when used in 10-13-10-11 mol/L concentrations. The effects of (-)BPAP on the release of both neurotransmitters were bell-shaped. We speculated that the rising phase of the concentration-effect curves was evoked by an agonist effect of (-)BPAP on TAAR1 whereas the declining phase was a result of heterodimerization of TAAR1 with pre- and postsynaptic dopamine D2 receptors. The bell-shaped curves suggest that the (-)BPAP-induced heterodimerization of TAAR1 with dopamine D2 receptors may switch off TAAR1 signaling and switch on transduction coupled to D2 receptors. We also suggest that (-)BPAP increases synaptic strength in a hypothetical quadrilateral neuronal organization consisting of dopaminergic nerve ending, GABAergic neurons, trace amine-producing D cells, and supportive glial cell processes.
Keywords: (-)BPAP; Dopaminergic activity enhancer effect; Rat striatum; Trace amines / Trace amine-associated receptor 1; [3H]Dopamine and [3H]GABA release.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing Interest: The authors declare no competing interests. Ethical Approval: All experimental procedures were approved by local Ethical Committees and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals, 8th Edition, 2011. Animal care and handling protocols were approved by the regional animal health authority in Hungary (Pest County Government Office, resolution number: PE/EA/285–5/2020, date: March 19, 2020). Consent to Participate: Not applicable.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

