Targeting fibroblast-endothelial cell interactions in LAM pathogenesis using 3D spheroid models and spatial transcriptomics
- PMID: 39903528
- PMCID: PMC11949067
- DOI: 10.1172/jci.insight.187899
Targeting fibroblast-endothelial cell interactions in LAM pathogenesis using 3D spheroid models and spatial transcriptomics
Abstract
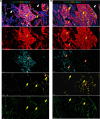
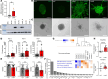
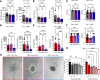
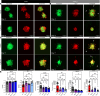
Lymphangioleiomyomatosis (LAM) is a progressive lung disease with limited treatments, largely because of an incomplete understanding of its pathogenesis. Lymphatic endothelial cells (LECs) invade LAM cell clusters, which include human melanoma black-45-positive epithelioid cells and smooth muscle α-actin-expressing LAM-associated fibroblasts (LAMFs). Recent evidence shows that LAMFs resemble cancer-associated fibroblasts, with LAMF-LEC interactions contributing to disease progression. To explore these mechanisms, we used spatial transcriptomics on LAM lung tissues and identified a gene cluster enriched in kinase signaling pathways linked to myofibroblasts and coexpressed with LEC markers. Kinase arrays revealed elevated PDGFR and FGFR in LAMFs. Using a 3D coculture spheroid model of primary LAMFs and LECs, we observed increased invasion in LAMF-LEC spheroids compared with non-LAM fibroblasts. Treatment with sorafenib, a multikinase inhibitor, significantly reduced invasion, outperforming rapamycin. We also verified tuberous sclerosis complex 2-deficient renal angiomyolipoma (TSC2-null AML) cells as key VEGF-A secretors; VEGF-A was suppressed by sorafenib in both TSC2-null AML cells and LAMFs. These findings highlight VEGF-A and basic FGF as potential therapeutic targets and suggest multikinase inhibition as a promising strategy for LAM.
Keywords: Cell biology; Cell migration/adhesion; Genetic diseases; Protein kinases; Pulmonology.
Conflict of interest statement
Figures





Update of
-
Targeting Fibroblast-Endothelial Interactions in LAM Pathogenesis: 3D Spheroid and Spatial Transcriptomic Insights for Therapeutic Innovation.bioRxiv [Preprint]. 2024 Sep 6:2023.06.12.544372. doi: 10.1101/2023.06.12.544372. bioRxiv. 2024. Update in: JCI Insight. 2025 Feb 04;10(6):e187899. doi: 10.1172/jci.insight.187899. PMID: 37398026 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

