Interim report on engineered NK cell trial in lung cancer refractory to immune checkpoint inhibitors
- PMID: 39903538
- PMCID: PMC11949060
- DOI: 10.1172/jci.insight.186890
Interim report on engineered NK cell trial in lung cancer refractory to immune checkpoint inhibitors
Abstract
Background: Non-small cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality, necessitating the exploration of alternate therapeutic approaches. Tumor-reactive or activated-by-cytokine killers (TRACK) are PD-L1+, highly cytolytic NK cells derived from umbilical cord blood NK cells and engineered to express soluble IL-15 (sIL15), and these cells show promise in preclinical studies against NSCLC.
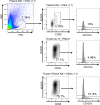
Methods: We assessed safety, persistence, homing, and cytotoxic activity in 6 patients with advanced, refractory, and progressing NSCLC who received a low dose of unmatched, allogeneic, off-the-shelf sIL15_TRACK NK cells. We evaluated NK cell presence and persistence with droplet digital PCR (ddPCR), flow cytometry, and immunofluorescence staining.
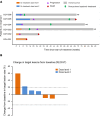
Results: sIL15_TRACK NK cells had peak measurements at 1 hour and became undetectable 4 hours after each infusion. Cognate ligands to activating NK cell receptors were found in NSCLC. sIL15_TRACK NK cells were observed in a lung tumor biopsy 7 days after the final infusion, confirming their sustainment and tumor-homing ability. They retained cytolytic function following isolation from the lung tumor. Three of 6 patients achieved disease stabilization on repeat imaging, while the others progressed.
Conclusion: Unmatched, allogeneic, cryopreserved, off-the-shelf sIL15_TRACK NK cells express activating receptors, home to tumor sites that express their cognate ligands, and retain cytolytic activity after infusion, underscoring their potential as a therapeutic approach in solid tumors. At low doses, the therapy was safely administered and showed preliminary evidence of activity in 3 of 6 patients with advanced and progressive NSCLC. Additional dose escalation cohorts and coadministration with atezolizumab are planned.
Clinicaltrials: gov NCT05334329.
Funding: Funding was provided by CytoImmune Therapeutics and grants from the National Cancer Institute (CA266457, CA033572, and CA210087).
Keywords: Clinical trials; Innate immunity; Lung cancer; NK cells; Oncology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

