Repetitive antigen stimulation in the periphery dictates the composition and recall responses of brain-resident memory CD8+ T cells
- PMID: 39903666
- PMCID: PMC11867863
- DOI: 10.1016/j.celrep.2025.115247
Repetitive antigen stimulation in the periphery dictates the composition and recall responses of brain-resident memory CD8+ T cells
Abstract
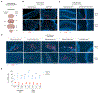
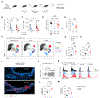
The human brain harbors virus-specific, tissue-resident memory (TRM) CD8+ T cells. However, the impact of repeated peripheral viral infection on the generation, phenotype, localization, and recall responses of brain TRM remains elusive. Here, utilizing two murine models of peripheral viral infection, we demonstrate that circulating memory CD8+ T cells with previous antigen exposure exhibit a markedly reduced capacity to form brain TRM compared to naive CD8+ T cells. Repetitively stimulated brain TRM also demonstrate differential inhibitory receptor expression, preserved functionality, and divergent localization patterns compared to primary memory counterparts. Despite these differences, repetitively stimulated brain TRM provide similar protection against intracranial infection as primary populations with superior recall-based recruitment of peripheral lymphocytes. As CD8+ T cells may distinctly seed the brain with each repeated infection of the same host, these findings point to heterogeneity in the brain TRM pool that is dictated by prior peripheral antigen stimulation history.
Keywords: CP: Immunology; CP: Neuroscience; brain T(RM); influenza virus; neuroimmunology; repetitive antigen stimulation; viral infection.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

