Exploring glycolytic enzymes in disease: potential biomarkers and therapeutic targets in neurodegeneration, cancer and parasitic infections
- PMID: 39904372
- PMCID: PMC11793985
- DOI: 10.1098/rsob.240239
Exploring glycolytic enzymes in disease: potential biomarkers and therapeutic targets in neurodegeneration, cancer and parasitic infections
Abstract
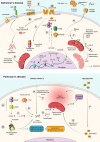
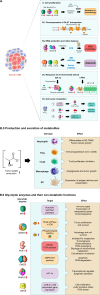
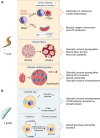
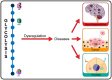
Glycolysis, present in most organisms, is evolutionarily one of the oldest metabolic pathways. It has great relevance at a physiological level because it is responsible for generating ATP in the cell through the conversion of glucose into pyruvate and reducing nicotinamide adenine dinucleotide (NADH) (that may be fed into the electron chain in the mitochondria to produce additional ATP by oxidative phosphorylation), as well as for producing intermediates that can serve as substrates for other metabolic processes. Glycolysis takes place through 10 consecutive chemical reactions, each of which is catalysed by a specific enzyme. Although energy transduction by glucose metabolism is the main function of this pathway, involvement in virulence, growth, pathogen-host interactions, immunomodulation and adaptation to environmental conditions are other functions attributed to this metabolic pathway. In humans, where glycolysis occurs mainly in the cytosol, the mislocalization of some glycolytic enzymes in various other subcellular locations, as well as alterations in their expression and regulation, has been associated with the development and progression of various diseases. In this review, we describe the role of glycolytic enzymes in the pathogenesis of diseases of clinical interest. In addition, the potential role of these enzymes as targets for drug development and their potential for use as diagnostic and prognostic markers of some pathologies are also discussed.
Keywords: diagnostic; glycolysis; pathogenesis of diseases; prognostic; therapeutic.
Conflict of interest statement
We declare we have no competing interests.
Figures

Similar articles
-
A mitochondrial carrier transports glycolytic intermediates to link cytosolic and mitochondrial glycolysis in the human gut parasite Blastocystis.Elife. 2024 May 23;13:RP94187. doi: 10.7554/eLife.94187. Elife. 2024. PMID: 38780415 Free PMC article.
-
Glycolytic enzymes in non-glycolytic web: functional analysis of the key players.Cell Biochem Biophys. 2024 Jun;82(2):351-378. doi: 10.1007/s12013-023-01213-5. Epub 2024 Jan 9. Cell Biochem Biophys. 2024. PMID: 38196050 Review.
-
Tumor glycolysis as a target for cancer therapy: progress and prospects.Mol Cancer. 2013 Dec 3;12:152. doi: 10.1186/1476-4598-12-152. Mol Cancer. 2013. PMID: 24298908 Free PMC article. Review.
-
Nicotinamide adenine dinucleotide (NAD+): essential redox metabolite, co-substrate and an anti-cancer and anti-ageing therapeutic target.Biochem Soc Trans. 2020 Jun 30;48(3):733-744. doi: 10.1042/BST20190033. Biochem Soc Trans. 2020. PMID: 32573651 Review.
-
Glycolysis-induced drug resistance in tumors-A response to danger signals?Neoplasia. 2021 Feb;23(2):234-245. doi: 10.1016/j.neo.2020.12.009. Epub 2021 Jan 6. Neoplasia. 2021. PMID: 33418276 Free PMC article. Review.
Cited by
-
TrypPROTACs Unlocking New Therapeutic Strategies for Chagas Disease.Pharmaceuticals (Basel). 2025 Jun 19;18(6):919. doi: 10.3390/ph18060919. Pharmaceuticals (Basel). 2025. PMID: 40573314 Free PMC article. Review.
-
Pyruvate kinase deficiency links metabolic perturbations to neurodegeneration and axonal protection.Mol Metab. 2025 Aug;98:102187. doi: 10.1016/j.molmet.2025.102187. Epub 2025 Jun 10. Mol Metab. 2025. PMID: 40505722 Free PMC article.
References
-
- Evans RD, Heather LC. 2019. Human metabolism: pathways and clinical aspects. Surgery 37, 302–309. (10.1016/j.mpsur.2019.03.006) - DOI
-
- van Steenwyk G, Jawaid A, Mansuy IM. 2019. Epigenetic inheritance of metabolic signals. In Nutritional epigenomics, pp. 167–190. San Diego, CA: Elsevier. (10.1016/b978-0-12-816843-1.00011-4) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

