Avoiding the tragedies of parasite tolerance in Darwinian beekeeping
- PMID: 39904384
- PMCID: PMC11793967
- DOI: 10.1098/rspb.2024.2433
Avoiding the tragedies of parasite tolerance in Darwinian beekeeping
Abstract
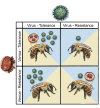
Bee declines have been partly attributed to the impacts of invasive or emerging parasite outbreaks. For western honeybees, Apis mellifera, major losses are associated with the virus-vectoring mite, Varroa destructor. In response, beekeepers have focused breeding efforts aimed at conferring resistance to this key parasite. One method of many is survival-based beekeeping where colonies that survive despite significant Varroa infestations produce subsequent colonies. We argue that this 'hands-off' approach will not always lead to Varroa resistance evolving but rather tolerance. Tolerance minimizes host fitness costs of parasitism without reducing parasite abundance, whereas resistance either prevents parasitism outright or keeps parasitism intensity low. With clear epidemiological distinctions, and as honeybee disease dynamics impact other wild bees owing to shared pathogens, we discuss why tolerance outcomes in honeybee breeding have important implications for wider pollinator health. Crucially, we argue that unintentional selection for tolerance will not only lead to more spillover from honeybees but may also select for pathogens that are more virulent in wild bees leading to 'tragedies of tolerance'. These tragedies can be avoided through successful breeding regimes that specifically select for low Varroa. We emphasize how insights from evolutionary ecology can be applied in ecologically responsible honeybee management.
Keywords: disease; honeybees; parasite; resistance; tolerance; varroa.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
-
- Gallai N, Salles JM, Settele J, Vaissière BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821. (10.1016/j.ecolecon.2008.06.014) - DOI
Publication types
MeSH terms
Grants and funding
- National Science Foundation Graduate Research Fellowship Program
- Georgia Beekeepers Association License Plate Proceeds Grants
- NC1173/Hatch Act 1887 Multi-State Research Fund
- 2023-51181-41246/U.S. Department of Agriculture's National Institute of Food and Agriculture - Specialty Crop Research Initiative
- 2011109/Division of Environmental Biology

