Phase I study of intratumoral administration of CV8102 in patients with advanced melanoma, squamous cell carcinoma of the skin, squamous cell carcinoma of the head and neck, or adenoid cystic carcinoma
- PMID: 39904560
- PMCID: PMC11795518
- DOI: 10.1136/jitc-2024-009352
Phase I study of intratumoral administration of CV8102 in patients with advanced melanoma, squamous cell carcinoma of the skin, squamous cell carcinoma of the head and neck, or adenoid cystic carcinoma
Abstract
Background: CV8102, a toll-like receptor 7/8 and RIG I agonist, has demonstrated antitumor immune responses in preclinical studies. We investigated intratumoral (IT) administration of CV8102 in patients with anti-programmed cell death protein-1 (PD-1) therapy-naïve or anti-PD-1 therapy-refractory cutaneous melanoma (cMEL) and in patients with advanced cutaneous squamous cell carcinoma, head and neck squamous cell carcinoma and adenoid cystic carcinoma.
Methods: This open-label, cohort-based, phase I dose escalation study aimed to establish the maximum tolerated dose (MTD), recommended dose (RD), safety and preliminary efficacy of CV8102 as monotherapy or in combination with a PD-1 inhibitor. The preliminary efficacy of the RD was assessed in patients with cMEL in the expansion cohorts.
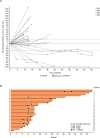
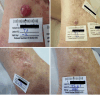
Results: Between September 2017 and October 2022, 98 patients were enrolled in monotherapy and combination therapy dose escalation and dose expansion cohorts. Two patients in the CV8102 monotherapy dose escalation cohort experienced relevant toxicities at the 900 µg dose level. One patient had Grade 3 aspartate transaminase/alanine aminotransferase elevation which met dose-limiting toxicity (DLT) criteria. Another patient experienced Grade 3 immune-mediated pneumonitis. No DLTs occurred in the combination therapy dose escalation cohort. The MTD was not formally reached and the RD for expansion was 600 µg. Common treatment-emergent adverse events were fever (57%), chills (37%) and fatigue (25%). In the dose escalation part, objective responses occurred in 3/33 patients treated with CV8102 as monotherapy and in 2/25 patients treated with CV8102 plus a PD-1 inhibitor. In the expansion cohorts in patients with anti-PD-1 therapy-refractory melanoma, 0/10 patients treated with CV8102 as monotherapy and 5/30 patients (17%) treated in combination with a PD-1 inhibitor experienced objective responses.
Conclusions: IT CV8102 was generally well tolerated with preliminary signs of efficacy as monotherapy and in combination with a PD-1 inhibitor.
Trial registration number: NCT03291002.
Keywords: Head and Neck Cancer; Immunotherapy; Intratumoral; Skin Cancer.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: TE and IT have received institutional funding for the work in this study. YS, IS, ME, LH, JK, AO, CW, PM, CLP, PB, MF, AP, MS and AS have no competing interests. CR is an occasional consultant for Roche, BMS, MSD, Sanofi, Pierre Fabre, AstraZeneca, and Novartis. CL has received consulting fees from BMS, MSD, Sanofi, Immunocore, and Pierre Fabre. JM-L has received consulting fees from Bristol-Myers Squibb, Highlight Therapeutics, Novartis, Pierre Fabre, Roche, and Sanofi. ER has received consulting fees from BMS, MSD, Novartis, and Pierre Fabre; speakers’ fees from Amgen, BMS, Delcath, MSD, Merck, Novartis, Pierre Fabre, Sanofi, Amgen, BMS, Curevac, Delcath, Incyte, MSD, Merck, Novartis, Pierre Fabre, Regeneron and Roche. PT has received consulting fees from Almirall, BMS, Kyowa Kirin, Merck, Novartis, Pierre Fabre, Sanofi, 4SC, and honorarium from Almirall, BMS, Biofrontera, Kyowa Kirin, Merck, Novartis, Pierre Fabre, Roche, Sanofi, and 4SC. BS-B, MG, JH, PW, TS, SDK, GQ, PC, MF, and UG-V are employees of CureVac SE. OS-K and SO have received consulting fees from CureVac SE.
Figures


References
-
- Zhang W, Zeng W, Jiang A, et al. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021;10:4905–22. doi: 10.1002/cam4.4046. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
