Determinants of 5-year survival in patients with advanced NSCLC with PD-L1≥50% treated with first-line pembrolizumab outside of clinical trials: results from the Pembro-real 5Y global registry
- PMID: 39904562
- PMCID: PMC11795382
- DOI: 10.1136/jitc-2024-010674
Determinants of 5-year survival in patients with advanced NSCLC with PD-L1≥50% treated with first-line pembrolizumab outside of clinical trials: results from the Pembro-real 5Y global registry
Abstract
Background: Pembrolizumab monotherapy is an established front-line treatment for advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) tumor proportion score (TPS)≥50%. However, real-world data on its long-term efficacy remains sparse.
Methods: This study assessed 5-year outcomes of first-line pembrolizumab monotherapy in a large, multicenter, real-world cohort of patients with advanced NSCLC and PD-L1 TPS≥50%, referred to as Pembro-real 5Y. Individual patient-level data (IPD) from the experimental arm of the KEYNOTE-024 trial were extracted (KN024 IPD cohort) to compare the long-term outcomes between the two cohorts. To further assess the reproducibility of clinical trial results, we reconstructed the "KN024 look-alike" cohort by excluding patients with an Eastern Cooperative Oncology Group-performance status (ECOG-PS)≥2, those requiring corticosteroids with doses ≥10 mg of prednisolone/equivalent, patients with positive/unknown epidermal growth factor receptor/anaplastic lymphoma kinase genotype, and those with pre-existing autoimmune disease. We additionally provided a hierarchical organization of determinants of long-term benefit through a conditional inference tree analysis.
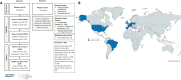
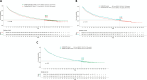
Results: The study included 1050 patients from 61 institutions across 14 countries, with a median follow-up of 70.3 months. The 5-year survival rate was 26.9% (95% CI: 23.8% to 30.2%), and median OS was 21.8 months (95% CI: 19.1 to 25.7), while 32 (3.0%) patients who achieved a complete response remained progression-free at the data cut-off. The KN024 look-alike cohort had a 5-year survival rate of 29.3% (95% CI: 25.5% to 33.6%) and a median OS of 27.5 months (95% CI: 22.8 to 31.3). Neither the overall study population nor the KN024 look-alike cohort exhibited significantly different OS compared with the KN024 IPD cohort. By the data cut-off, 1015 patients (96.7%) had permanently discontinued treatment: 659 (64.9%) due to progressive disease, 156 (15.4%) due to toxicity, 77 (7.6%) due to treatment completion, and 106 (10.4%) due to other reasons. Overall, 222 participants (21.1%) were treated for a minimum period of 24 months, among them the 5-year survival rates were: 31.7%, 72.7%, 78.6%, 84.2% for patients who discontinued treatment due to progressive disease, toxicity, treatment completion, and other reasons, respectively.
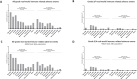
Conclusion: This study provides valuable real-world evidence that confirms the long-term efficacy of pembrolizumab outside of clinical trials. Hierarchical organization indicates ECOG-PS, age and PD-L1-TPS as the most important predictors of 5-year survival, potentially informing clinical practice.
Keywords: Immune Checkpoint Inhibitor; Immunotherapy; Lung Cancer.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AC received grants for consultancies/advisory boards from MSD, BMS, OncoC4, IQVIA, AstraZeneca, REGENERON, Access Infinity, Ardelis Health, Alpha Sight, Guidepoint, Roche; speaker fees from AstraZeneca, Pierre-Fabre, MSD, Sanofi/REGENERON; payment for writing/editorial activity from BMS, MSD; travel support from Sanofi/REGENERON, MSD. JB declares honoraria/consulting or advisory role from AstraZeneca, BMS, Roche, Access Oncology, travel support from MSD, Roche, Janssen Oncology. GPS has received payment or honoraria for advisory boards from Novartis, Roche, Bayer, unrelated to this project. DO’R has received conference attendance support from Takeda, Janssen, Servier, MSD. EB has received grants or contracts from AstraZeneca, Roche and honoraria for lectures from Merck-Sharp & Dome, AstraZeneca, Pfizer, Eli-Lilly, Bristol Myers Squibb, Novartis, Takeda and Roche; EB has been member of Data Safety Monitoring Board or Advisory Board of Merck-Sharp & Dome, Pfizer, Novartis, Bristol Myers Squibb, AstraZeneca, Celltrion and Roche. AA declares consulting or advisory role for Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Roche, MSD, Pfizer, Eli Lilly, Astellas, Takeda, and Amgen; speaker’s bureau for Eli Lilly, and AstraZeneca. AR has received advisory board or speaker bureau honoraria from AstraZeneca, MSD, Novartis, Pfizer, BMS, Takeda, and Amgen; compensated activity for editorial projects from AstraZeneca, MSD, Novartis, Roche, and Regeneron. AL has received speakers’ fee for AstraZeneca, MSD, Sanofi and Takeda; he also received travel support from MSD and Novartis, has been on advisory board for AstraZeneca, BeiGene, Novartis and Sanofi, and has attended editorial activities sponsored by Eli Lilly and Roche. FM received honorary for advisory board roles with MDS, BMS, Takeda, Roche, AstraZeneca, Novartis. Paolo Bironzo served as consultant/advisory board for Regeneron, Pierre-Fabre, Janssen, Seagen. DO declares research funding/grants (to institution) from BMS, Merck, Palobiofarma, Pfizer, Genentech, AstraZeneca, Nuvalent, AbbVie, Onc.AI. TN-D received support to attend educational conferences from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Lilly, MSD, Otsuka, Roche, Takeda; advisory roles for AbbVie, Amgen, Bayer, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Eli-Lilly, EQRx, Gilead, GSK, Janssen, Merck, MSD, Novartis, Novocure, Otsuka, Pfizer, Roche, Sanofi, Takeda; speaker bureau from Amgen, AstraZeneca, Chugai, Gilead, Janssen, Lilly, Medscape, Guardant, Merck, MSD, Roche, Takeda; trial steering committees’ member for AstraZeneca, Roche. BT received lecture fees from Pfizer. LC is an employee of Fortrea. BT declares honoraria from Roche. IM declares travel support from Takada, MSD, Pfizer, Oxyvie and speaker fees from Regeneron. A-MD declares research grants from Amgen, the Dutch Cancer Society and HANART, consulting fees from Amgen, Bayer, Boehringer Inglheim, Sanofy, Roche, Janssen and AstraZeneca, speaker fees from Janssen, Pfizer, AstraZeneca, Lilly and Takeda, advisory board role for Takeda and Roche. GLR declares fees for advisory boards, travel support, consultancies from MSD, BMS, Roche, Sanofi, Regeneron, Lilly, AstraZeneca, Janssen, Pfizer, Novartis, Bayer, Takeda, Amgen, GSK, Daichii. TAH declares stock interests for GlaxoSmithKline and honoraria from Novartis. BR served as consultant/advisory board for AMGEN, Regeneron, AstraZeneca, Capvision. Speaker fee: AstraZeneca. Received honoraria from Targeted Oncology, SITC. All other authors declare no conflicts of interest associated with the present study.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
