NADPH-mediated seedless in situ formation of gold or gold-platinum nanoparticles for the enzymatic determination of atropine
- PMID: 39904770
- PMCID: PMC11794361
- DOI: 10.1007/s00604-025-06964-x
NADPH-mediated seedless in situ formation of gold or gold-platinum nanoparticles for the enzymatic determination of atropine
Abstract
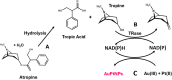
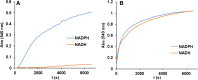
The without-seeds in situ formation of gold nanoparticles from NADPH and its application to the colorimetric determination of atropine (a tropane alkaloid) in cereals is reported. The method is based on a chemical hydrolysis, followed by an enzymatic oxidation by NADP catalyzed by tropinone reductase in the presence of Au(III) or Au(III)/Pt(II). During this reaction, the formed NADPH reduces the metal ion precursor to AuNPs (or AuPtNPs) and the absorption due to the plasmon band (550 nm or 575 nm) is measured. The method (AuPtNPs) allows the determination of the analyte in the concentration range 0.025 to 0.09 mM with an RSD of 3% (n = 5) and is applied to its determination in spiked buckwheat samples using the standard addition method, with 96.9 ± 2.0% recovery. It is also demonstrated that NAD(P)H and NADH have different kinetics for AuNP generation, which could be used to discriminate between these two cofactors.
Keywords: Gold nanoparticles; Gold-platinum nanoparticles; NADH; Tropane alkaloid; Tropinone reductase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Gold nanoparticle formation as an indicator of enzymatic methods: colorimetric L-phenylalanine determination.Anal Bioanal Chem. 2022 Mar;414(8):2641-2649. doi: 10.1007/s00216-022-03900-3. Epub 2022 Jan 21. Anal Bioanal Chem. 2022. PMID: 35064303 Free PMC article.
-
Colorimetric enzymatic rapid test for the determination of atropine in baby food using a smartphone.Anal Bioanal Chem. 2024 Dec;416(30):7317-7323. doi: 10.1007/s00216-024-05401-x. Epub 2024 Jul 3. Anal Bioanal Chem. 2024. PMID: 38960939 Free PMC article.
-
Colorimetric and electrochemical (dual) thrombin assay based on the use of a platinum nanoparticle modified metal-organic framework (type Fe-MIL-88) acting as a peroxidase mimic.Mikrochim Acta. 2019 Jan 10;186(2):94. doi: 10.1007/s00604-018-3209-4. Mikrochim Acta. 2019. PMID: 30631938
-
Colorimetric-enzymatic determination of tyramine by generation of gold nanoparticles.Mikrochim Acta. 2020 Feb 18;187(3):174. doi: 10.1007/s00604-020-4141-y. Mikrochim Acta. 2020. PMID: 32072299
-
Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric in Vitro Diagnostics.Nano Lett. 2017 Sep 13;17(9):5572-5579. doi: 10.1021/acs.nanolett.7b02385. Epub 2017 Aug 18. Nano Lett. 2017. PMID: 28813601
Cited by
-
Kinetic and analytical characterization of a new tropinone oxidase enzyme and its application to the simultaneous determination of the tropane alkaloids atropine and scopolamine.Anal Bioanal Chem. 2025 Jun;417(14):3169-3176. doi: 10.1007/s00216-025-05856-6. Epub 2025 Apr 10. Anal Bioanal Chem. 2025. PMID: 40208267 Free PMC article.
References
-
- Aldossary SA (2022) Review on pharmacology of atropine, clinical use and toxicity. Biomed Pharmacol J 15:691–197. 10.13005/bpj/2408
-
- Adamse P, van Egmond HP, Noordam MY, Mulder PPJ, De Nijs M (2014) Tropane alkaloids in food: poisoning incidents. Qual Assur Saf Crop Foods 6:15–24. 10.3920/QAS2013.0314
-
- EFSA Panel on Contaminants in the Food Chain (2013) Scientific Opinion on Tropane alkaloids in food and feed. EFSA J 11:3386. 10.2903/j.efsa.2013.3386
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
