High-altitude hypoxia aggravated neurological deficits in mice induced by traumatic brain injury via BACH1 mediating astrocytic ferroptosis
- PMID: 39905004
- PMCID: PMC11794473
- DOI: 10.1038/s41420-025-02337-8
High-altitude hypoxia aggravated neurological deficits in mice induced by traumatic brain injury via BACH1 mediating astrocytic ferroptosis
Abstract
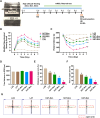
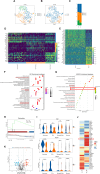
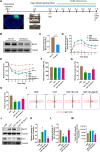
Traumatic brain injury (TBI) is one of the leading causes of disability and mortality, which was classified as low-altitude TBI and high-altitude TBI. A large amount of literature shows that high-altitude TBI is associated with more severe neurological impairments and higher mortality rates compared to low-altitude TBI, due to the special environment of high-altitude hypoxia. However, the role of high-altitude hypoxia in the pathogenesis of TBI remains unclear. In order to deeply investigate this scientific issue, we constructed a high-altitude hypoxic TBI model at different altitudes and used animal behavioral assessments (Modified neurological severity score, rotarod test, elevated plus maze test) as well as histopathological analyses (brain gross specimens, brain water content, Evans blue content, hypoxia inducible factor-1α, Hematoxylin-Eosin staining and ROS detection) to reveal its underlying principles and characteristics. We found that with higher altitude, TBI-induced neurological deficits were more severe and the associated histopathological changes were more significant. Single-nuclear RNA sequencing was subsequently employed to further reveal differential gene expression profiles in high-altitude TBI. We found a significant increase in ferroptosis of astrocytes in cases of high-altitude TBI compared to those at low-altitude TBI. Analyzing transcription factors in depth, we found that Bach1 plays a crucial role in regulating key molecules that induce ferroptosis in astrocytes following high-altitude TBI. Down-regulation of Bach1 can effectively alleviate high-altitude TBI-induced neurological deficits and histopathological changes in mice. In conclusion, high-altitude hypoxia may significantly enhance the ferroptosis of astrocytes and aggravate TBI by up-regulating Bach1 expression. Our study provides a theoretical foundation for further understanding of the mechanism of high-altitude hypoxic TBI and targeted intervention therapy.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Air Force Medical University (approval No. 20210419) on April 19, 2021, and conducted following the instructions of Declaration of Helsinki of the World Medical Association. Informed consent was obtained from all participants.
Figures



References
-
- Liu Y, Zhao Z, Guo J, Ma Y, Li J, Ji H, et al. Anacardic acid improves neurological deficits in traumatic brain injury by anti-ferroptosis and anti-inflammation. Exp Neurol. 2023;370:114568. - PubMed
-
- Tang Y, Liu Y, Zhou H, Lu H, Zhang Y, Hua J, et al. Esketamine is neuroprotective against traumatic brain injury through its modulation of autophagy and oxidative stress via AMPK/mTOR-dependent TFEB nuclear translocation. Exp Neurol. 2023;366:114436. - PubMed
-
- Wei L, Zhang J, Zhang B, Geng J, Tan Q, Wang L, et al. Complement C3 participates in the function and mechanism of traumatic brain injury at simulated high altitude. Brain Res. 2020;1726:146423. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

