Implantation of engineered adipocytes suppresses tumor progression in cancer models
- PMID: 39905264
- PMCID: PMC12319119
- DOI: 10.1038/s41587-024-02551-2
Implantation of engineered adipocytes suppresses tumor progression in cancer models
Abstract
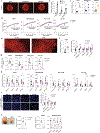
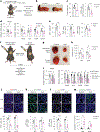
Tumors exhibit an increased ability to obtain and metabolize nutrients. Here, we implant engineered adipocytes that outcompete tumors for nutrients and show that they can substantially reduce cancer progression, a technology termed adipose manipulation transplantation (AMT). Adipocytes engineered to use increased amounts of glucose and fatty acids by upregulating UCP1 were placed alongside cancer cells or xenografts, leading to significant cancer suppression. Transplanting modulated adipose organoids in pancreatic or breast cancer genetic mouse models suppressed their growth and decreased angiogenesis and hypoxia. Co-culturing patient-derived engineered adipocytes with tumor organoids from dissected human breast cancers significantly suppressed cancer progression and proliferation. In addition, cancer growth was impaired by inducing engineered adipose organoids to outcompete tumors using tetracycline or placing them in an integrated cell-scaffold delivery platform and implanting them next to the tumor. Finally, we show that upregulating UPP1 in adipose organoids can outcompete a uridine-dependent pancreatic ductal adenocarcinoma for uridine and suppress its growth, demonstrating the potential customization of AMT.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.A. is a cofounder and on the scientific advisory board of Regel Therapeutics. N.A. receives funding from BioMarin Pharmaceutical Incorporate. H.P.N. and N.A. have filed a patent application covering embodiments and concepts disclosed in the paper. The other authors declare no competing interests.
Figures










References
Grants and funding
- R01 CA281361/CA/NCI NIH HHS/United States
- R01 DK124769/DK/NIDDK NIH HHS/United States
- 1R01CA283826/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- 1R01DK124769/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- SFSU EDUC2-12693/California Institute for Regenerative Medicine (CIRM)
- 1P50CA168504/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- 1K99HG012576/U.S. Department of Health & Human Services | NIH | National Human Genome Research Institute (NHGRI)
- R01 CA283826/CA/NCI NIH HHS/United States
- K99 HG012576/HG/NHGRI NIH HHS/United States
- R01 CA292019/CA/NCI NIH HHS/United States
- EDUC4-12812/California Institute for Regenerative Medicine (CIRM)
- P50 CA168504/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

