Six Injections of Modified Adjuvanted PQ Grass Is Effective and Well-Tolerated in a Pivotal Phase III Trial
- PMID: 39905623
- PMCID: PMC12261879
- DOI: 10.1111/all.16491
Six Injections of Modified Adjuvanted PQ Grass Is Effective and Well-Tolerated in a Pivotal Phase III Trial
Abstract
Background: PQ Grass 27600 SU (PQ Grass) cumulative dose is a pre-seasonal, six-injection, aluminium-free, modified subcutaneous immunotherapy product under development for the treatment of allergic rhinitis (AR). A pivotal Phase III randomised double-blind, placebo-controlled clinical trial was performed to evaluate the efficacy and safety of PQ Grass in subjects with seasonal AR.
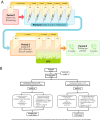
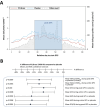
Methods: An adaptive group sequential trial PQGrass306 (G306) with one pre-defined interim analysis was designed, using 2 parallel groups applying a 1:1 active versus placebo randomisation of patients aged 18-65. The primary efficacy endpoint was the EAACI (European Academy of Allergy and Clinical Immunology) Combined Symptom and Medication Score (EAACI-CSMS0-6) averaged over the peak grass pollen season (GPS).
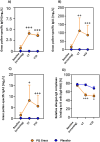
Results: 858 subjects were screened and 555 subjects were randomised. Based on the results of the pre-defined interim analysis, the trial was stopped for success showing superiority in favour of PQ Grass. The primary endpoint EAACI-CSMS0-6 (peak GPS) demonstrated a highly significant and clinically meaningful point difference of PQ Grass over placebo of -0.27 points (95% CI: -0.42 to -0.12), corresponding to a relative difference of -20.3% (p = 0.0005). Highly consistent and beneficial results were obtained for PQ Grass for all key secondary endpoints. Significant induction of blocking IgG4 and IgA antibody subclasses occurred. PQ Grass was well tolerated, and no unexpected safety signals occurred.
Conclusions: This pivotal Phase III trial demonstrated a significant and clinically meaningful effect on the primary endpoint as well as highly consistent secondary endpoint results and a supportive safety profile.
Keywords: allergic rhinoconjunctivitis; grass pollen allergy; pivotal Phase III clinical trial; short‐course treatment; subcutaneous immunotherapy.
© 2025 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
S.Z. has received personal fees for lectures from Engelhard GmbH, from Böhringer Ingelheim, Allergy Therapeutics, AstraZeneca, SanofiAventis, EryDel SpA and Lofarma during the last 3 years. J.A.B. is acting as consultant and PI for: Allergy Therapeutics, Stallergens, ALK, Eli Lilly, Novartis, Genentech, Sanofi Regeneron, AZ, GSK, Amgen. G.J.S. reports grants from ALK‐Abelló, personal fees from ALK‐Abelló, personal fees from Allergopharma, personal fees from Novartis, and personal fees from Stallergenes‐Greer, outside the submitted work. M.J. reports personal fees from ALK‐Abello, Allergopharma, Stallergenes, Anergis, Allergy Therapeutics, Leti, HAL, during the conduct of the study; personal fees from GSK, Novartis, Teva, Takeda, Chiesi outside the submitted work; and is the Allergy Journal Deputy Editor. O.P. reports grants for his institution as clinical trial unit for RESONATE from Allergy Therapeutics Plc, UK, and he reports grants and/or personal fees and/or travel support from ALK‐Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Laboratorios LETI/LETI Pharma, GlaxoSmithKline, ROXALL Medizin, Novartis, Sanofi‐Aventis and Sanofi‐Genzyme, Med Update Europe GmbH, streamedup! GmbH, Pohl‐Boskamp, Inmunotek S.L., John Wiley and Sons/AS, Paul‐Martini‐Stiftung (PMS), Regeneron Pharmaceuticals Inc., RG Aerztefortbildung, Institut für Disease Management, Springer GmbH, AstraZeneca, IQVIA Commercial, Ingress Health, Wort & Bild Verlag, Verlag ME, Procter & Gamble, ALTAMIRA, Meinhardt Congress GmbH, Deutsche Forschungsgemeinschaft, Thieme, Deutsche AllergieLiga e.V., AeDA, Alfried‐Krupp Krankenhaus, Red Maple Trials Inc., Königlich Dänisches Generalkonsulat, Medizinische Hochschule Hannover, ECM Expro & Conference Management, Technical University Dresden, Lilly, Japanese Society of Allergy, Forum für Medizinische Fortbildung, Dustri‐Verlag, Pneumolive, ASIT Biotech, LOFARMA, Almirall, Paul‐Ehrlich‐Institut, outside the submitted work; and he is Vice President of the EAACI and member of EAACI Excom, member of ext. board of directors DGAKI; coordinator, main‐ or co‐author of different position papers and guidelines in rhinology, allergology and allergen‐immunotherapy; he is associate editor (AE) of Allergy and Clinical Translational Allergy. R.M. reports grants and personal fees from Allergy Therapeutics Ltd., during the conduct of the study; personal fees from ALK, grants from ASIT biotech, personal fees from allergopharma, grants and personal fees from Bencard, grants from Leti, grants, personal fees and non‐financial support from Lofarma, non‐financial support from Roxall, grants and personal fees from Stallergenes, grants from Optima, personal fees from Friulchem, personal fees from Hexal, personal fees from Servier, personal fees from Klosterfrau, non‐financial support from Atmos, personal fees from Bayer, non‐financial support from Bionorica, personal fees from FAES, personal fees from GSK, personal fees from MSD, personal fees from Johnson & Johnson, personal fees from Meda, personal fees and non‐financial support from Novartis, non‐financial support from Otonomy, personal fees from Stada, personal fees from UCB, non‐financial support from Ferrero, grants from Hulka, personal fees from Nuvo, grants and personal fees from Ursapharm, personal fees from Menarini, personal fees from Mundipharma, personal fees from Pohl‐Boskamp, grants from Inmunotek, grants from Cassella‐med GmbH & Co. KG, personal fees from Laboratoire de la Mer, personal fees from Sidroga, grants and personal fees from HAL BV, personal fees from Lek, personal fees from PRO‐AdWise, personal fees from Angelini Pharma, grants and non‐financial support from JGL, grants and personal fees from bitop, grants from Sanofi, grants and personal fees from Probelte Pharma, grants from Diater, personal fees from Worg Pharmaceuticals, outside the submitted work. M.H.S. reports research grants from Immune Tolerance Network, Medical Research Council, Allergy Therapeutics, LETI Laboratorios, Rovolo Biotherapeutics and lecture fees from Allergy Therapeutics and Leti Laboratorios, all outside the submitted work. M.B. and U.E.B. reports grants and/or personal fees and/or travel support from AZ Pollen Research GmbH, outside the submitted work. L.D. acts as a consultant for Allergy Therapeutics and a Consultant and/or Speakers Bureau for GSK, Sanofi, Regeneron, Astra Zeneca, ALK‐Abello, Amgen, Bryn Pharm, Areteia. J.A.L. reports grants via Biomedical Research Funding (Imperial College BRC), all outside the submitted work. L.K. has received research grants from Allergy Therapeutics/Bencard, Great Britain/Germany; ALK‐Abelló, Denmark; Allergopharma, Germany; Aimmune, USA; ASIT Biotech, Belgium; AstraZeneca, Sweden, Bionorica, Germany; BioNTech, Germany, Biomay, Austria, Boehringer Ingelheim, Germany, Circassia, USA; Chiesi, Italy; Cytos, Switzerland; Curalogic, Denmark; HAL, Netherlands; Lofarma, Italy; Menarini, Italy; Viatris/Mylan, USA; Novartis, Switzerland, Leti, Spain; ROXALL, Germany; GlaxoSmithKline (GSK), Great Britain; Sanofi, France; Stallergenes, France; Thermofisher, USA and/or has served on the speaker's bureau or was consulting for the above mentioned pharmaceutical companies. L.K. is the current President of German Society of Allergology AeDA, Governance Committee Board Member of the European Academy for Allergy and Clinical Immunology (EAACI), Vice‐President of German Academy for Allergy and Environmental Medicine and Editor‐in‐Chief of AllergoJournal and AllergoJournal International. M.O. received personal fees and/or speakers' honoraria from Allergy Therapeutics/Bencard Allergie GmbH, Thermo Fisher Scientific, Hycor Diagnostics, as well as grants from the European Commission, the National Research Fund Luxembourg (FNR) and Angany, all outside the submitted work. M.S., M.F.K., and P.‐J.K. are employees of Allergy Therapeutics (UK) Plc. and are named inventors on patents related to PQ Grass.
Figures
References
-
- Roberts G., Pfaar O., Akdis C. A., et al., “EAACI Guidelines on Allergen Immunotherapy: Allergic Rhinoconjunctivitis,” Allergy 73, no. 4 (2018): 765–798. - PubMed
-
- Jutel M., Agache I., Bonini S., et al., “International Consensus on Allergen Immunotherapy II: Mechanisms, Standardization, and Pharmacoeconomics,” Journal of Allergy and Clinical Immunology 137, no. 2 (2016): 358–368. - PubMed
-
- Arshad S. H., “Does Allergen Immunotherapy for Allergic Rhinitis Prevent Asthma?,” Annals of Allergy, Asthma & Immunology 129, no. 3 (2022): 286–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

