MiniMed 780G system performance in older users with type 1 diabetes: Real-world evidence and the case for stricter glycaemic targets
- PMID: 39905659
- PMCID: PMC11885105
- DOI: 10.1111/dom.16227
MiniMed 780G system performance in older users with type 1 diabetes: Real-world evidence and the case for stricter glycaemic targets
Abstract
Aims: Large-scale studies on the effectiveness of automated insulin delivery (AID) systems in older people with type 1 diabetes are still limited. A multinational, retrospective, real-world study was conducted to examine the performance of the MiniMed™ 780G advanced hybrid closed-loop system in users with type 1 diabetes aged ≥56 years compared with those aged 16-55 years.
Materials and methods: Data from 35 366 MiniMed™ 780G system users aged 16-55 years and 7415 users aged ≥56 years were included. The main outcome was time in range 70-180 mg/dL (TIR); other continuous glucose monitoring (CGM) metrics were also assessed.
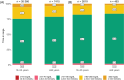
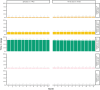
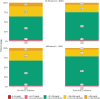
Results: Across all users, mean TIR was 77.1% for users aged ≥56 years and 73.1% for those aged 16-55 years (Δ4.0, 95% confidence interval [CI]: 3.8-4.2, p <0.0001). In users employing the optimal system settings (i.e., Glucose Target: 100 mg/dL; active insulin time: 2 h), mean TIR was 81.9% in older and 79.7% in younger users (Δ2.2, 95% CI: 1.5-2.9, p <0.0001). Across all users, mean time below range <70 mg/dL (TBR70) was 1.5% in older and 2.1% in younger users. In older users, TIR and TBR70 remained consistent over 12 months.
Conclusions: This real-world analysis demonstrated that older MiniMed™ 780G system users with type 1 diabetes can achieve a TIR >70% without increasing hypoglycaemia risk. Users employing optimal settings showed the best outcomes. The system performed as well as or better than in younger users. These findings support the case that more stringent TIR targets can be achieved safely.
Keywords: continuous glucose monitoring (CGM); database research; insulin pump therapy; real‐world evidence; type 1 diabetes.
© 2025 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
SH has received consultancy/speaking fees from Novo Nordisk, Eli Lily, Zealand, Mylan and Medtronic and research support from Dexcom. DO has received consultancy/speaking fees from Medtronic, Insulet, Abbott, Novo and Sanofi; research support from Medtronic, Insulet, Dexcom, Roche, GlySense, BioCapillary and Endogenex; and served on advisory boards for Medtronic, Insulet, Abbott, Ypsomed, Novo Nordisk and Sanofi. JJ served as speaker and/or member of the advisory board for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic, Nordic InfuCare, Novo Nordisk and Sanofi. TC has received consultancy/speaking fees from Eli Lilly, Sanofi, MSD, Novo Nordisk, Medtronic, Geffen Medical, AZ and BI and research support from Medtronic, MSD and Novo Nordisk. VS, AA, JC, TH and OC are employees of Medtronic.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

