Direct-acting antiviral therapy for patients with hepatitis C virus-related hepatocellular carcinoma: A nationwide cohort study
- PMID: 39905837
- PMCID: PMC12260621
- DOI: 10.3350/cmh.2024.1015
Direct-acting antiviral therapy for patients with hepatitis C virus-related hepatocellular carcinoma: A nationwide cohort study
Abstract
Background/aims: The survival benefit of direct-acting antiviral (DAA) therapy for hepatitis C virus (HCV) infection in patients with hepatocellular carcinoma (HCC), particularly in Barcelona Clinic Liver Cancer (BCLC) stages B/C, remains largely uncertain. We aimed to explore the impact of DAA therapy on overall survival (OS) in HCC patients using a nationwide cohort study.
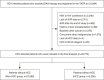
Methods: We utilized the nationwide Taiwan Association for the Study of the Liver (TASL) HCV Registry (TACR) database to include all adults receiving a DAA therapy for HCV, excluding those with other viral infections, liver transplantation, non-HCC malignancies, and terminal-staged HCC. We respectively analyzed the adjusted odds ratio (aOR) for sustained virological response (SVR) and adjusted hazard ratio (aHR) for OS.
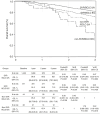
Results: Between December 2013 and December 2020, 2,205 (9.3%) patients with HCC and 21,569 (90.7%) patients without HCC were include. The SVR rates were 96.6% in the HCC group and 98.8% in the non-HCC group (P<0.001), with HCC being an independent risk factor affecting SVR (aOR 0.41; 95% CI 0.31-0.54; P<0.001). In the whole patient cohort, SVR was independently associated with improved OS (aHR 0.46; 95% CI 0.35-0.60; P<0.001). Among patients with baseline HCC, SVR remained an independent factor related to OS (aHR 0.41; 95% CI 0.28-0.59; P<0.001). The impact of SVR on OS persisted significantly across BCLC stages 0/A and stages B/C.
Conclusion: High SVR rates among HCC patients underscore the importance of DAA therapy in enhancing OS, reaffirming its efficacy across various HCC stages.
Keywords: Antivirals; Chronic hepatitis C; Liver cancer; Survival; Sustained virological response.
Conflict of interest statement
TY Lee: research support from Gilead, Merck and Roche diagnostics; consultant of BMS, Gilead, and Astra- Zeneca and speaker of Abbvie, BMS, Eisai, Gilead, Roche and AstraZeneca.
ML Yu: research support from Abbvie, Abbott Diagnostic, BMS, Gilead, Merck and Roche diagnostics; consultant of Abbvie, Abbott Diagnostic, BMS, Gilead, Roche and Roche diagnostics and speaker of Abbvie, BMS, Eisai, Gilead, Roche and Roche diagnostics. All other authors declare that they do not have any relevant conflict of interest.
Figures



References
-
- Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Rete Sicilia Selezione Terapia–HCV (RESIST-HCV) Incidence of hepatocellular carcinoma in patients with HCVassociated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421.e4. - PubMed
-
- Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Rete Sicilia Selezione Terapia – HCV (RESISTHCV) and Italian Liver Cancer (ITA.LI.CA.) Group Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265–273. - PubMed
MeSH terms
Substances
Grants and funding
- Kaohsiung Medical University
- MOHW111-TDU-B-221-114007/Ministry of Health and Welfare
- MOHW112-TDU-B-221-124007/Ministry of Health and Welfare
- MOHW113-TDU-B-221-134007/Ministry of Health and Welfare
- NSTC112-2314-B-075A-010-MY3/National Science and Technology Council
- TCVGH-1123301C/Taichung Veterans General Hospital
- TCVGH-1133301C/Taichung Veterans General Hospital
- VTA112-V1-3-3/Taichung Veterans General Hospital
- VTA113-V1-1-2/Taichung Veterans General Hospital
- KMU-NSTC 112-2635-B-037-001-MY2/Kaohsiung Medical University
- KMU-MOST 111-2314-B-037-069-MY2/Kaohsiung Medical University
- MOHW113-TDU-B-221-134007/Kaohsiung Medical University
- KMUH112-2R09/Kaohsiung Medical University Hospital
- KMUH111-1R04/Kaohsiung Medical University Hospital
- KMUH-DK(A)113002/Kaohsiung Medical University Hospital
LinkOut - more resources
Full Text Sources
Medical

