Rhopalurus junceus scorpion venom induces G2/M cell cycle arrest and apoptotic cell death in human non-small lung cancer cell lines
- PMID: 39906356
- PMCID: PMC11792888
- DOI: 10.1590/1678-9199-JVATITD-2024-0035
Rhopalurus junceus scorpion venom induces G2/M cell cycle arrest and apoptotic cell death in human non-small lung cancer cell lines
Abstract
Background: Non-small cell lung cancers (NSCLC) represent the primary cause of cancer-related deaths worldwide. Rhopalurus junceus venom has been shown to exert cytotoxic effects against a panel of epithelial cancer cells in vitro and suggested that NSCLC was the subtype most susceptible to the treatment.
Methods: This study evaluated the effect of Rhopalurus junceus scorpion venom on cell viability, in non-cancerous (MRC-5, lung; CHO-K1, ovary) and NSCLC (A549; NCI-H460) cell lines. The effects on cell cycle, apoptosis, and cell signaling-related proteins were determined by flow cytometry and WB. Protein fractions responsible for the observed effect were identified using HPLC.
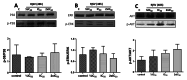
Results: Scorpion venom was more effective against NSCLC than non-cancerous cells. Emax values were 20.0 ± 5.8% and 22.47 ± 6.02% in A549 and NCI-H460 cancer cells, respectively, as compared to 50 ± 8.1% in MRC-5 and 54.99 ± 7.39% in CHO-K1 cells. It arrested NSCLC cells in the G2/M phase, while non-cancerous cells were arrested in the S (MRC-5) or G0/G1 (CHO-K1) phases. No changes were observed in the Bax/Bcl-2 or the cleaved-caspase 3/Total caspase 3 ratios in cells treated with venom. Likewise, the scorpion venom treatment did not affect p-ERK, p-AKT, or p-38MAPK protein levels. In contrast, scorpion venom treatment increased the cytosolic apoptosis-inducing factor (AIF) in A549 cells, indicating caspase-independent apoptosis. Additionally, combined etoposide/venom exposure provoked G2/M arrest and apoptosis in NSCLC more strongly than either substance alone. Furthermore, upon crude venom fractioning through RP-HPLC, we found two soluble fractions with high cytotoxic effects.
Conclusion: The present study concludes that a specific fraction of Rhopalurus junceus venom reduces cell viability of NSCLC cells. The AIF protein plays a key role in mediating caspase-independent apoptotic cell death. These findings suggest that Rhopalurus junceus venom enhances the anticancer effect of etoposide in vitro by causing cell cycle arrest and caspase-independent apoptosis.
Keywords: Apoptosis; Cell cycle arrest; Rhopalurus junceus; Scorpion venom; Synergism.
Conflict of interest statement
Competing interests: AD-G and AG works for LifEscozul Chile SpA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
