Mutual coupling of neurons in the circadian master clock: What we can learn from fruit flies
- PMID: 39906412
- PMCID: PMC11791320
- DOI: 10.1016/j.nbscr.2025.100112
Mutual coupling of neurons in the circadian master clock: What we can learn from fruit flies
Abstract
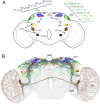
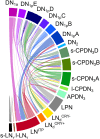
Circadian master clocks in the brain consist of multiple neurons that are organized into populations with different morphology, physiology, and neuromessenger content and presumably different functions. In most animals, these master clocks are distributed bilaterally, located in close proximity to the visual system, and synchronized by the eyes with the light-dark cycles of the environment. In mammals and cockroaches, each of the two master clocks consists of a core region that receives information from the eyes and a shell region from which most of the output projections originate, whereas in flies and several other insects, the master clocks are distributed in lateral and dorsal brain regions. In all cases, morning and evening clock neurons seem to exist, and the communication between them and other populations of clock neurons, as well as the connection across the two brain hemispheres, is a prerequisite for normal rhythmic function. Phenomena such as rhythm splitting, and internal desynchronization are caused by the "decoupling" of the master clocks in the two brain hemispheres or by the decoupling of certain clock neurons within the master clock of one brain hemisphere. Since the master clocks in flies contain relatively few neurons that are well characterized at the individual level, the fly is particularly well suited to study the communication between individual clock neurons. Here, we review the organization of the bilateral master clocks in the fly brain, with a focus on synaptic and paracrine connections between the multiple clock neurons, in comparison with other insects and mammals.
Keywords: Clock neurons; Drosophila melanogaster; Dual oscillator model; Flywire connectome; Multi-oscillator system; Neuropeptides.
© 2025 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

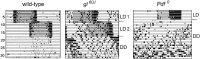

Similar articles
-
The circadian clock in the brain: a structural and functional comparison between mammals and insects.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004 Aug;190(8):601-13. doi: 10.1007/s00359-004-0527-2. Epub 2004 May 20. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004. PMID: 15156341 Review.
-
Real time, in vivo measurement of neuronal and peripheral clocks in Drosophila melanogaster.Elife. 2022 Oct 3;11:e77029. doi: 10.7554/eLife.77029. Elife. 2022. PMID: 36190119 Free PMC article.
-
Connectomic analysis of the Drosophila lateral neuron clock cells reveals the synaptic basis of functional pacemaker classes.Elife. 2022 Jun 29;11:e79139. doi: 10.7554/eLife.79139. Elife. 2022. PMID: 35766361 Free PMC article.
-
Reconfiguration of a Multi-oscillator Network by Light in the Drosophila Circadian Clock.Curr Biol. 2018 Jul 9;28(13):2007-2017.e4. doi: 10.1016/j.cub.2018.04.064. Epub 2018 Jun 14. Curr Biol. 2018. PMID: 29910074 Free PMC article.
-
The lateral and dorsal neurons of Drosophila melanogaster: new insights about their morphology and function.Cold Spring Harb Symp Quant Biol. 2007;72:517-25. doi: 10.1101/sqb.2007.72.063. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419311 Review.
Cited by
-
Insect circadian plasticity as a proposed target for the expression of parasite extended phenotypes.NPJ Biol Timing Sleep. 2025;2(1):29. doi: 10.1038/s44323-025-00046-0. Epub 2025 Aug 1. NPJ Biol Timing Sleep. 2025. PMID: 40755487 Free PMC article. Review.
-
Circadian Vesicle Capture and Phase-Delayed Activity Maximize Synaptic Neuropeptide Release by Clock Neurons.bioRxiv [Preprint]. 2025 Feb 25:2023.12.01.569590. doi: 10.1101/2023.12.01.569590. bioRxiv. 2025. PMID: 38106047 Free PMC article. Preprint.
-
Sexually dimorphic and clock gene-specific effects of artificial light at night on Drosophila behavioural rhythms.Proc Biol Sci. 2025 Aug;292(2053):20251170. doi: 10.1098/rspb.2025.1170. Epub 2025 Aug 27. Proc Biol Sci. 2025. PMID: 40858251 Free PMC article.
-
Synaptic Targets of Circadian Clock Neurons Influence Core Clock Parameters.bioRxiv [Preprint]. 2025 Feb 6:2025.01.30.635801. doi: 10.1101/2025.01.30.635801. bioRxiv. 2025. PMID: 39975067 Free PMC article. Preprint.
-
Circadian rhythms are more resilient to pacemaker neuron disruption in female Drosophila.PLoS Biol. 2025 May 6;23(5):e3003146. doi: 10.1371/journal.pbio.3003146. eCollection 2025 May. PLoS Biol. 2025. PMID: 40327674 Free PMC article.
References
-
- Atkinson S.E., Maywood E.S., Chesham J.E., Wozny C., Colwell C.S., Hastings M.H., Williams S.R. Cyclic AMP signaling control of action potential firing rate and molecular circadian pacemaking in the suprachiasmatic nucleus. J. Biol. Rhythm. 2011;26:210–220. doi: 10.1177/0748730411402810. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

