A classic key innovation constrains oral jaw functional diversification in fishes
- PMID: 39906576
- PMCID: PMC11790220
- DOI: 10.1093/evlett/qrae046
A classic key innovation constrains oral jaw functional diversification in fishes
Abstract
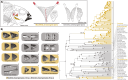
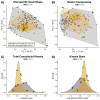
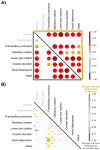
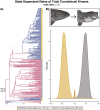
Modifications to the pharyngeal jaws-a prey processing system located posterior to the mouth cavity-are widely considered a key innovation that enhanced diversification within several prominent fish clades. Seen in cichlids, damselfishes, wrasses, and a few other lineages, these musculoskeletal alterations are believed to increase the evolutionary independence and, thus, the diversification of the oral and pharyngeal jaw systems. To test this classic hypothesis, we conducted comparative phylogenetic analyses to assess the effect of the pharyngeal novelty on the diversification of feeding morphology and kinematics across a taxonomically diverse sample of spiny-rayed fishes. We quantified movements of the oral jaws and other craniofacial structures from 689 suction-feeding strikes using high-speed videos collected from 228 species with and without the pharyngeal jaw novelty. Contradicting long-held predictions, we find significantly greater disparity across all traits and faster rates of oral jaw functional evolution in fishes without the specialized prey processing system. The modified pharyngeal jaw is undoubtedly a functional innovation as it enhances the strength of the prey processing system, facilitating exceptional transition rates to feeding on hard and tough prey. However, it also restricts the diversification of the feeding system, revealing that the impact of pharyngognathy is more nuanced than previously thought. In light of these and other recent findings, a reinterpretation of the macroevolutionary consequences of the pharyngeal jaw novelty is needed.
Keywords: Acanthomorpha; evolutionary integration; feeding kinematics; geometric morphometrics; morphology; pharyngognathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Society for the Study of Evolution (SSE) and European Society for Evolutionary Biology (ESEB).
Figures

Similar articles
-
The evolution of pharyngognathy: a phylogenetic and functional appraisal of the pharyngeal jaw key innovation in labroid fishes and beyond.Syst Biol. 2012 Dec 1;61(6):1001-27. doi: 10.1093/sysbio/sys060. Epub 2012 Jun 27. Syst Biol. 2012. PMID: 22744773
-
The cichlid pharyngeal jaw novelty enhances evolutionary integration in the feeding apparatus.Evolution. 2023 Sep 1;77(9):1917-1929. doi: 10.1093/evolut/qpad109. Evolution. 2023. PMID: 37326103
-
Head Shape Modulates Diversification of a Classic Cichlid Pharyngeal Jaw Innovation.Am Nat. 2019 Nov;194(5):693-706. doi: 10.1086/705392. Epub 2019 Sep 13. Am Nat. 2019. PMID: 31613667
-
Functional Innovations and the Conquest of the Oceans by Acanthomorph Fishes.Curr Biol. 2017 Jun 5;27(11):R550-R557. doi: 10.1016/j.cub.2017.03.044. Curr Biol. 2017. PMID: 28586692 Review.
-
The Teleost Intramandibular Joint: A mechanism That Allows Fish to Obtain Prey Unavailable to Suction Feeders.Integr Comp Biol. 2015 Jul;55(1):85-96. doi: 10.1093/icb/icv042. Epub 2015 May 21. Integr Comp Biol. 2015. PMID: 26002346 Review.
Cited by
-
Phylogenomics establishes an Early Miocene reconstruction of reef vertebrate diversity.Sci Adv. 2025 May 9;11(19):eadu6149. doi: 10.1126/sciadv.adu6149. Epub 2025 May 7. Sci Adv. 2025. PMID: 40333985 Free PMC article.
-
Incompatibility between two major innovations shaped the diversification of fish feeding mechanisms.PLoS Biol. 2025 Jun 24;23(6):e3003225. doi: 10.1371/journal.pbio.3003225. eCollection 2025 Jun. PLoS Biol. 2025. PMID: 40554461 Free PMC article.
References
-
- Adams, D. C. (2014a). A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution, 68(9), 2675–2688. https://doi.org/10.1111/evo.12463 - DOI - PubMed
-
- Adams, D. C. (2014b). Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Systematic Biology, 63(2), 166–177. https://doi.org/10.1093/sysbio/syt105 - DOI - PubMed
-
- Adams, D. C., & Collyer, M. L. (2015). Permutation tests for phylogenetic comparative analyses of high-dimensional shape data: What you shuffle matters. Evolution, 69(3), 823–829. https://doi.org/10.1111/evo.12596 - DOI - PubMed
-
- Adams, D. C., & Collyer, M. L. (2016). On the comparison of the strength of morphological integration across morphometric datasets. Evolution, 70(11), 2623–2631. https://doi.org/10.1111/evo.13045 - DOI - PubMed
-
- Adams, D. C., & Collyer, M. L. (2018). Multivariate phylogenetic comparative methods: Evaluations, comparisons, and recommendations. Systematic Biology, 67(1), 14–31. https://doi.org/10.1093/sysbio/syx055 - DOI - PubMed

