Targeting the Gut-Heart Axis Improves Cardiac Remodeling in a Clinical Scale Model of Cardiometabolic Syndrome
- PMID: 39906593
- PMCID: PMC11788474
- DOI: 10.1016/j.jacbts.2024.09.004
Targeting the Gut-Heart Axis Improves Cardiac Remodeling in a Clinical Scale Model of Cardiometabolic Syndrome
Abstract
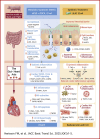
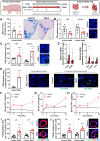
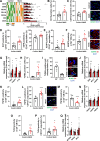
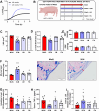
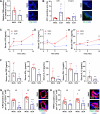
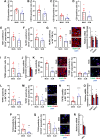
Poor diet, gut dysbiosis, and systemic inflammation constitute a gut-heart axis (GHA) that may affect heart failure with preserved ejection fraction. Clinical scale models to interrogate this axis are limited. Here, we show the full extent of the GHA-gut barrier function loss, systemic and microvascular inflammation, and its colocalization with apoptosis (left atrium) and hypertrophy (left ventricle). Gut barrier function primacy in regulating the GHA is supported by a synbiotic intervention that shuts down gut epithelial permeability, markedly decreasing systemic inflammation and, remarkably, cardiac structural changes in left heart chambers. These data support a new paradigm for targeting heart failure with preserved ejection fraction via the GHA.
Keywords: heart failure; metabolic syndrome; microbiome; synbiotic.
© 2025 The Authors.
Conflict of interest statement
This study was funded by a grant from Tate and Lyle Solutions USA LLC and by a grant from Science Foundation Ireland to the Alimentary Pharmabiotic Centre at University College Cork, grant number 12/RC/2273-P2. Drs Herisson, Karnik, Laurie, Canene-Adams, Ross, Stanton, Caplice are named inventors on intellectual property co-owned by University College Cork and Tate and Lyle Solutions, LLC relating to synbiotic treatment of metabolic syndrome. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
Comment in
-
Synbiotics and Gut-Heart Axis in Cardiometabolic Disease.JACC Basic Transl Sci. 2025 Jan 27;10(1):16-19. doi: 10.1016/j.jacbts.2024.11.008. eCollection 2025 Jan. JACC Basic Transl Sci. 2025. PMID: 39958467 Free PMC article.
Similar articles
-
A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation.J Am Coll Cardiol. 2013 Jul 23;62(4):263-71. doi: 10.1016/j.jacc.2013.02.092. Epub 2013 May 15. J Am Coll Cardiol. 2013. PMID: 23684677 Review.
-
Gut microbiome of a porcine model of metabolic syndrome and HF-pEF.Am J Physiol Heart Circ Physiol. 2020 Mar 1;318(3):H590-H603. doi: 10.1152/ajpheart.00512.2019. Epub 2020 Feb 7. Am J Physiol Heart Circ Physiol. 2020. PMID: 32031871
-
Interactions between the gut microbiome, associated metabolites and the manifestation and progression of heart failure with preserved ejection fraction in ZSF1 rats.Cardiovasc Diabetol. 2024 Aug 14;23(1):299. doi: 10.1186/s12933-024-02398-6. Cardiovasc Diabetol. 2024. PMID: 39143579 Free PMC article.
-
Advances in extracardiac mechanisms for heart failure with preserved ejection fraction.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 Dec 28;47(12):1733-1739. doi: 10.11817/j.issn.1672-7347.2022.220310. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 36748385 Free PMC article. Chinese, English.
-
Clinical Phenotypes of Heart Failure With Preserved Ejection Fraction to Select Preclinical Animal Models.JACC Basic Transl Sci. 2022 May 25;7(8):844-857. doi: 10.1016/j.jacbts.2021.12.009. eCollection 2022 Aug. JACC Basic Transl Sci. 2022. PMID: 36061340 Free PMC article. Review.
Cited by
-
Meta-inflammation and endotoxemia in a highly translational porcine model of diet-induced obesity.Lab Anim (NY). 2025 Aug 6. doi: 10.1038/s41684-025-01588-3. Online ahead of print. Lab Anim (NY). 2025. PMID: 40770069
-
Synbiotics and Gut-Heart Axis in Cardiometabolic Disease.JACC Basic Transl Sci. 2025 Jan 27;10(1):16-19. doi: 10.1016/j.jacbts.2024.11.008. eCollection 2025 Jan. JACC Basic Transl Sci. 2025. PMID: 39958467 Free PMC article.
-
Lactobacillus Restructures the Micro/Mycobiome to Combat Inflammation-Mediated Right Ventricular Dysfunction in Pulmonary Arterial Hypertension.Circ Heart Fail. 2025 Jul;18(7):e012524. doi: 10.1161/CIRCHEARTFAILURE.124.012524. Epub 2025 May 16. Circ Heart Fail. 2025. PMID: 40376801
References
-
- Borlaug B.A., Sharma K., Shah S.J., Ho J.E. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810–1834. - PubMed
-
- Usman M.S., Siddiqi T.J., Anker S.D., et al. Effect of SGLT2 inhibitors on cardiovascular outcomes across various patient populations. J Am Coll Cardiol. 2023;81(25):2377–2387. - PubMed
-
- Murphy S.P., Kakkar R., McCarthy C.P., Januzzi J.L. Inflammation in heart failure: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(11):1324–1340. - PubMed
LinkOut - more resources
Full Text Sources

