Structure simulation-based comparison of active site variations in fungal ornithine decarboxylases
- PMID: 39906711
- PMCID: PMC11792860
- DOI: 10.1080/19420889.2025.2458872
Structure simulation-based comparison of active site variations in fungal ornithine decarboxylases
Abstract
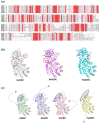
Polyamines play crucial roles in various biological processes, including cell proliferation and differentiation, immune response modulation, and signal transduction. Ornithine decarboxylase (ODC) initiates polyamine biosynthesis by catalyzing the conversion of ornithine to putrescine in a pyridoxal phosphate (PLP)-dependent manner. While the structures of mammalian and protozoan ODCs have been elucidated, fungal ODCs remain uncharacterized. In this study, AlphaFold2 was employed to simulate the structures of ODCs from four fungi: Kluyveromyces lactis, Candida albicans, Debaryomyces hansenii, and Schizosaccharomyces pombe. The results indicated that, although these ODCs share α/β-barrel and β-sheet domains, their active site conformations exhibit subtle differences. Additionally, substrate selectivity among ODCs and related decarboxylases varied depending on the distance between the Cα of aspartate or glutamate residues within the specificity helix and the C4α of PLP. Notably, the bacterial Campylobacter jejuni decarboxylase (CjCANSDC), which binds the largest substrate, exhibits the longest distance, whereas fungal ODC, which binds the smallest substrate, displays the shortest distance. Furthermore, significant differences in the composition of amino acid residues within the active sites were also observed. This study provides insights into the structural diversity and catalytic activity of ODCs across a broad range of organisms, advancing the understanding of structure-dependent evolutionary processes.
Keywords: Candida albicans; Debaryomyces hansenii; Kluyveromyces lactis; PLP-dependent enzyme; Schizosaccharomyces pombe; ornithine decarboxylase; polyamine; specificity helix.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
