Crystal structure of AlPCl8
- PMID: 39906786
- PMCID: PMC11789175
- DOI: 10.1107/S2056989024010661
Crystal structure of AlPCl8
Abstract
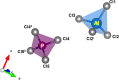
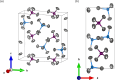
The crystal structure of aluminium phospho-rus chloride (systematic name: phospho-rus tetra-chloride tetra-chloridoa-luminate), (PCl4)[AlCl4] or AlPCl8, was determined and refined using single-crystal X-ray diffraction data. The compound crystallizes in the ortho-rhom-bic space group Pbcm. The asymmetric unit comprises one Al atom, one P atom, and five Cl atoms. The structure is characterized by isolated AlCl4 and PCl4 tetra-hedra, isostructural with FePCl8 and GaPCl8.
Keywords: aluminium phosphorus chloride; aluminium(III) chloride; crystal structure; phosphorus(V) chloride; single-crystal.
© Seo et al. 2024.
Figures
References
-
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst.36, 1487.
-
- Bruker (2006). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Chen, H., Wong, L. L. & Adams, S. (2019). Acta Cryst. B75, 18–33. - PubMed
-
- Fischer, W. & Jübermann, O. (1938). Z. Anorg. Allg. Chem.235, 337–351.
-
- Kistenmacher, T. J. & Stucky, G. D. (1968). Inorg. Chem.7, 2150–2155.
LinkOut - more resources
Full Text Sources
