Computational design, expression, and characterization of a Plasmodium falciparum multi-epitope, multi-stage vaccine candidate (PfCTMAG)
- PMID: 39906795
- PMCID: PMC11791285
- DOI: 10.1016/j.heliyon.2025.e42014
Computational design, expression, and characterization of a Plasmodium falciparum multi-epitope, multi-stage vaccine candidate (PfCTMAG)
Abstract
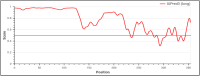
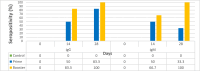
Malaria, a tropical disease, claims the lives of thousands of people annually and the development of resistance to insecticides and antimalarial drugs poses a great challenge to current prevention and control strategies. Current malaria vaccines are limited in efficacy, duration of protection, and safety, due to the high antigenic diversity and complex life cycle of the Plasmodium parasite. This study sought to design and assess a more effective multi-stage, multi-epitope vaccine candidate for the control of malaria. A multi-epitope malaria vaccine candidate was designed in silico using multiple antigens from both the pre-erythrocytic and erythrocytic stages, expressed in bacteria, and its sero-reactivity to antibodies in plasma from malaria-positive (cases) and negative individuals (controls) was assessed using enzyme-linked immunosorbent assay (ELISA). Immunization experiments were equally conducted with BALB/c mice. In-silico analysis revealed that the designed antigen, PfCTMAG (Plasmodium falciparum Circumsporozoite, Thrombospondin-related adhesion protein, Merozoite surface protein 2, Apical asparagine (Asn)-rich protein and Glutamate-Rich Protein), effectively bound to Toll-like receptor 4 (TLR-4) and triggered a strong immune response. In sero-reactivity studies, malaria-positives (cases) had higher anti-PfCTMAG IgG ( p = 0.024) and IgM ( p < 0.001) levels compared to malaria negatives (controls). The mice immunized with PfCTMAG did not show adverse reactions and had higher levels of IgG antibodies (p = 0.002) compared to controls, thereby validating the safety and immunogenicity of PfCTMAG as a promising vaccine candidate.
Keywords: Immunogenicity; In silico; Multi-epitope; Sero-reactivity; Vaccine candidate.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
PfSPZ Vaccine induces focused humoral immune response in HIV positive and negative Tanzanian adults.EBioMedicine. 2024 Oct;108:105364. doi: 10.1016/j.ebiom.2024.105364. Epub 2024 Sep 30. EBioMedicine. 2024. PMID: 39353279 Free PMC article.
-
Immunogenicity of a chimeric Plasmodium falciparum merozoite surface protein vaccine in Aotus monkeys.Malar J. 2016 Mar 15;15:159. doi: 10.1186/s12936-016-1226-5. Malar J. 2016. PMID: 26975721 Free PMC article.
-
Designing a multi-epitope vaccine against blood-stage of Plasmodium falciparum by in silico approaches.J Mol Graph Model. 2020 Sep;99:107645. doi: 10.1016/j.jmgm.2020.107645. Epub 2020 May 15. J Mol Graph Model. 2020. PMID: 32454399
-
Towards an RTS,S-based, multi-stage, multi-antigen vaccine against falciparum malaria: progress at the Walter Reed Army Institute of Research.Vaccine. 2005 Mar 18;23(17-18):2243-50. doi: 10.1016/j.vaccine.2005.01.142. Vaccine. 2005. PMID: 15755604 Review.
-
Current Challenges in the Identification of Pre-Erythrocytic Malaria Vaccine Candidate Antigens.Front Immunol. 2020 Feb 21;11:190. doi: 10.3389/fimmu.2020.00190. eCollection 2020. Front Immunol. 2020. PMID: 32153565 Free PMC article. Review.
Cited by
-
New therapeutic strategies for malaria.Biophys Rev. 2025 Mar 8;17(2):701-707. doi: 10.1007/s12551-025-01296-9. eCollection 2025 Apr. Biophys Rev. 2025. PMID: 40376415 Review.
-
One Health Approach to the Computational Design of a Lipoprotein-Based Multi-Epitope Vaccine Against Human and Livestock Tuberculosis.Int J Mol Sci. 2025 Feb 13;26(4):1587. doi: 10.3390/ijms26041587. Int J Mol Sci. 2025. PMID: 40004053 Free PMC article.
References
-
- World Health Organization . License: CC BY-NC-SA 3.0 IGO; Geneva: 2022. World Malaria Report 2022.
-
- Fairhurst R.M., Nayyar G.M.L., Breman J.G., Hallett R., Vennerstrom J.L., Duong S., Ringwald P., Wellems T.E., Plowe C.V., Dondorp A.M. Artemisinin-resistant malaria: research challenges, opportunities, and public health implications. Am. J. Trop. Med. Hyg. 2012;87(2):231–241. doi: 10.4269/ajtmh.2012.12-0025. - DOI - PMC - PubMed
-
- Aguttu C., Okech B.A., Mukisa A., Lubega G.W. Screening and characterization of hypothetical proteins of Plasmodium falciparum as novel vaccine candidates in the fight against malaria using reverse vaccinology. J. Genet. Eng. Biotechnol. 2021;19(1):103. doi: 10.1186/s43141-021-00199-y. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

