Small extracellular vesicles derived from miRNA-486 overexpressed dental pulp stem cells mitigate high altitude pulmonary edema through PTEN/PI3K/AKT/eNOS pathway
- PMID: 39906863
- PMCID: PMC11791212
- DOI: 10.1016/j.heliyon.2025.e41960
Small extracellular vesicles derived from miRNA-486 overexpressed dental pulp stem cells mitigate high altitude pulmonary edema through PTEN/PI3K/AKT/eNOS pathway
Abstract
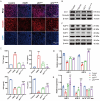
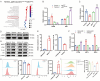
High altitude pulmonary edema (HAPE) is a life-threatening, non-cardiogenic pulmonary edema characterized by rapid onset and high mortality. Extracellular vesicles of mesenchymal stem cells are used in the treatment of a variety of lung diseases, but their use in HAPE remains underreported. This study explores the therapeutic potential of miRNA-486 modified extracellular vesicles from dental pulp stem cells (sEVmiR-486) against HAPE, aiming to decipher the associated molecular mechanisms. The rat HAPE model was established by exposing subjects to a simulated high-altitude, low-oxygen environment within a specialized chamber. The HAPE-afflicted rats received sEVNull and sEVmiR-486 intravenously, and the therapeutic effect was assessed through histopathological analysis, pulmonary artery pressure, lung water content, as well as markers of oxidative stress and inflammation. To supplement in vivo findings, pulmonary microvascular endothelial cells (PMVEC) were stressed with cobalt chloride to emulate hypoxic damage, and then treated with sEVNull and sEVmiR-486 to unravel the mechanism of action. The sEVNull mitigated pathological changes in the lungs, reduced pulmonary artery pressure and lung water content, and alleviated oxidative stress and inflammatory responses in cases of HAPE. Moreover, sEVNull enhanced vascular reactivity and restored pulmonary permeability and tight junction integrity, these effects were intensified by miRNA-486 overexpression. Notably, sEVmiR-486 attenuated oxidative damage in hypoxic PMVEC cells by modulating the PTEN/PI3K/Akt/eNOS signaling pathway. miRNA-486 fortified DPSC-sEVs intervention as a novel and potent treatment strategy for HAPE.
Keywords: Extracellular vesicles; High-altitude pulmonary edema; Oxidative stress; miR-486.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Superoxide dismutase 1-modified dental pulp stem cells alleviate high-altitude pulmonary edema by inhibiting oxidative stress through the Nrf2/HO-1 pathway.Gene Ther. 2024 Jul;31(7-8):422-433. doi: 10.1038/s41434-024-00457-x. Epub 2024 Jun 4. Gene Ther. 2024. PMID: 38834681
-
Susceptibility to high-altitude pulmonary edema is associated with circulating miRNA levels under hypobaric hypoxia conditions.Am J Physiol Lung Cell Mol Physiol. 2020 Aug 1;319(2):L360-L368. doi: 10.1152/ajplung.00168.2020. Epub 2020 Jun 17. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32692577
-
Eleutheroside B Pretreatment Attenuates Hypobaric Hypoxia-Induced High-Altitude Pulmonary Edema by Regulating Autophagic Flux via the AMPK/mTOR Pathway.Phytother Res. 2024 Dec;38(12):5657-5671. doi: 10.1002/ptr.8333. Epub 2024 Sep 22. Phytother Res. 2024. PMID: 39307910
-
[Pathophysiology, prevention and therapy of altitude pulmonary edema].Schweiz Med Wochenschr. 1992 Aug 4;122(31-32):1151-8. Schweiz Med Wochenschr. 1992. PMID: 1496342 Review. German.
-
High altitude pulmonary edema: a pressure-induced leak.Respir Physiol Neurobiol. 2007 Sep 30;158(2-3):266-73. doi: 10.1016/j.resp.2007.05.002. Epub 2007 May 18. Respir Physiol Neurobiol. 2007. PMID: 17602898 Review.
Cited by
-
Advances in stem cell-based therapeutics for acute high-altitude illness: research progress and prospects.Front Physiol. 2025 Aug 1;16:1614098. doi: 10.3389/fphys.2025.1614098. eCollection 2025. Front Physiol. 2025. PMID: 40821940 Free PMC article. Review.
References
-
- Luks A.M., Beidleman B.A., Freer L., Grissom C.K., Keyes L.E., McIntosh S.E., Rodway G.W., Schoene R.B., Zafren K., Hackett P.H. Wilderness medical society clinical practice guidelines for the prevention, diagnosis, and treatment of acute altitude illness: 2024 update. Wilderness Environ. Med. 2024;35(1_suppl):2S–19S. doi: 10.1016/j.wem.2023.05.013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

