Placental trophoblast aging in advanced maternal age is related to increased oxidative damage and decreased YAP
- PMID: 39906872
- PMCID: PMC11790555
- DOI: 10.3389/fcell.2025.1479960
Placental trophoblast aging in advanced maternal age is related to increased oxidative damage and decreased YAP
Abstract
Introduction: The advanced maternal age (AMA) pregnancies escalate rapidly, which are frequently linked to higher risks of adverse outcomes. Advanced maternal age (AMA) placenta exhibited premature aging, presumably resulting in trophoblast dysfunction, inadequate placentation. However, the precise reasons and mechanisms of trophoblast aging in AMA placenta remain unclear, posing a significant limitation to provide effective guidance for prenatal healthcare in clinical settings. Notably, the organism shows heightened vulnerability to oxidative damage as it ages. YAP (Yes-associated protein) was reported to play a critical role in regulation of aging and resisting oxidative damage, yet these roles had not been elucidated in the placenta. Therefore, this study explored the relationship between trophoblast cell aging and oxidative injury and YAP in AMA pregnancy, which not only provided an insight into the mechanisms of trophoblast cell aging, but also provide valuable directions for healthcare during AMA pregnancy.
Methods: In this study, human term placentas were collected from AMA and normal pregnancies for the analysis of aging, oxidative damage and YAP level. HTR8/SVneo cells were manipulated with (hydrogen peroxide) H2O2 to explore the effects of oxidative damage on trophoblast cell senescence and YAP levels. YAP expression in HTR8/SVneo cells was manipulated to investigate its role in trophoblastic senescence and oxidative damage.
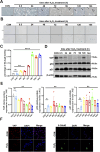
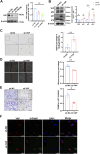
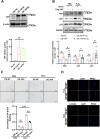
Results: Compared with the control group, the AMA placenta exhibits increased aging biomarkers, which is coupled with an elevation in oxidative damage within placental trophoblast cells and a notable decline in YAP levels. Cellular experiments demonstrated that oxidative damage from H2O2 triggered trophoblast cell senescence and resulted in a reduction of YAP levels. Furthermore, employing molecular modification to silence YAP expression in these cells led to an induction of aging. Conversely, overexpressing YAP ameliorated both trophoblast cell aging and the associated DNA oxidative damage that arised from H2O2.
Conclusion: The decline of YAP in AMA pregnancy should be responsible for the increased oxidative injury and premature placenta aging, indicating that YAP plays a significant role in combating oxidative damage and delaying aging, thereby providing a new guidance for prenatal care in AMA pregnancies. Maintaining YAP levels or implementing anti-oxidative stress interventions could potentially mitigate the incidence of complications involved AMA pregnancy.
Keywords: DNA oxidative damage; YAP; advanced maternal age; pregnancy complication; trophoblast aging.
Copyright © 2025 Guo, Pan, Chen, Huang, Li, Gou and Gao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation.Aging Cell. 2021 Oct;20(10):e13491. doi: 10.1111/acel.13491. Epub 2021 Oct 3. Aging Cell. 2021. PMID: 34605151 Free PMC article.
-
Reduced cell invasion may be a characteristic of placental defects in pregnant women of advanced maternal age at single-cell level.J Zhejiang Univ Sci B. 2022 Sept 15;23(9):747-759. doi: 10.1631/jzus.B2101024. J Zhejiang Univ Sci B. 2022. PMID: 36111571 Free PMC article.
-
Advanced maternal age causes premature placental senescence and malformation via dysregulated α-Klotho expression in trophoblasts.Aging Cell. 2021 Jul;20(7):e13417. doi: 10.1111/acel.13417. Epub 2021 Jun 9. Aging Cell. 2021. PMID: 34105233 Free PMC article.
-
YAP-mediated trophoblast dysfunction: the common pathway underlying pregnancy complications.Cell Commun Signal. 2023 Dec 14;21(1):353. doi: 10.1186/s12964-023-01371-2. Cell Commun Signal. 2023. PMID: 38098027 Free PMC article. Review.
-
TGFβ signalling: a nexus between inflammation, placental health and preeclampsia throughout pregnancy.Hum Reprod Update. 2024 Jul 1;30(4):442-471. doi: 10.1093/humupd/dmae007. Hum Reprod Update. 2024. PMID: 38519450 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

